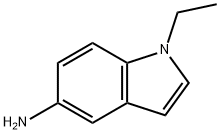
1H-Indol-5-amine,1-ethyl-(9CI) synthesis
- Product Name:1H-Indol-5-amine,1-ethyl-(9CI)
- CAS Number:220844-49-5
- Molecular formula:C10H12N2
- Molecular Weight:160.22

193977-99-0
5 suppliers
inquiry

220844-49-5
10 suppliers
inquiry
Yield:220844-49-5 98.2%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethyl acetate at 50; under 760.051 Torr; for 12 h;
Steps:
4 4.1.4 1-Ethyl-1H-indol-5-amine (4)
Into a two-necked flask, previously dried in an oven, about 0.300g of Pd/C 10% and approximately 50mL of ethyl acetate were placed. After connecting the flask to an elastomer balloon containing hydrogen gas, the mixture was stirred at room temperature for 1h in order to saturate the suspension of Pd/C with hydrogen. Then, 1.69g (8.90mmol) of compound 1 in 15mL of ethyl acetate was added dropwise to the suspension, and the mixture was stirred under hydrogen at atmospheric pressure and heated by means of an oil bath at 50-60°C while monitoring the progress of the reaction by TLC analysis (ethyl acetate/toluene/n-hexane, 1:1:1). At the end of the reaction, the mixture was filtered through a celite pad, and the solution was concentrated under vacuum to give 1.40g of amine as a dark liquid. Yield: 98.2%; Rf: 0.28 (toluene/n-hexane/ethyl acetate, 1:1:1); 1H NMR (300MHz, DMSO-d6): δ 7.16 (m, 2H, H-3 e H-7), 6.69 (d, J=2.28Hz, 1H, H-4), 6.54 (dd, J=8.5, 2.28Hz, 1H, H-6), 6.15 (d, J=3.05Hz, 1H, H-2), 4.48 (s br, 2H, NH2), 4.06 (q, J=7.3Hz, 2H, CH2), 1.55 (t, J=7.3Hz, 3H, CH3) ppm; HRMS (ESI-MS, 140eV): m/z [M+H]+ calculated for C10H13N2+, 161.2231; found, 161.1593.
References:
Bortolozzi, Roberta;Carta, Davide;Dal Prà, Matteo;Antoniazzi, Giuseppe;Mattiuzzo, Elena;Sturlese, Mattia;Di Paolo, Veronica;Calderan, Laura;Moro, Stefano;Hamel, Ernest;Quintieri, Luigi;Ronca, Roberto;Viola, Giampietro;Ferlin, Maria Grazia [European Journal of Medicinal Chemistry,2019,vol. 178,p. 297 - 314]
6146-52-7
458 suppliers
$13.00/1g

220844-49-5
10 suppliers
inquiry