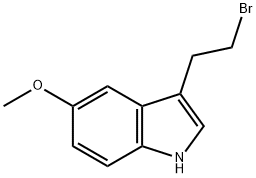
1H-INDOLE,3-(2-BROMOETHYL)-5-METHOXY- synthesis
- Product Name:1H-INDOLE,3-(2-BROMOETHYL)-5-METHOXY-
- CAS Number:18334-96-8
- Molecular formula:C11H12BrNO
- Molecular Weight:254.12
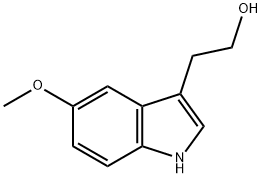
712-09-4
133 suppliers
$28.00/25mg

18334-96-8
9 suppliers
inquiry
Yield:18334-96-8 73.8%
Reaction Conditions:
with phosphorus tribromide in diethyl ether at 0; for 12 h;
Steps:
2.1.3. 3-(2-bromoethyl)-5-methoxy-1H-indole (fragment 2)
The phosphorus tribromide (1.51 g, 5.6 mmol) was added dropwiseto the solution of 7 (1.52 g, 8.0 mmol) in anhydrous diethyl ether (30mL) at 0 C, and the resulting mixture was stirred for 12 h. After completion, the solution was slowly quenched by water (30 mL) and extracted by ethyl acetate (3 × 30 mL). The combined organic layers were washed with saturated brine and dried over by Na2SO4, after filtration, eliminated under reduced pressure. The crude brown oil was purified by chromatography column (petroleum ether/EtOAc, 20:1) toafford the fragment 2 as a yellow oil (1.49 g, 73.8 %). 1H NMR (600MHz, DMSO-d6), δ (ppm): 10.76 (s, 1H), 7.23 (d, J = 8.7 Hz, 1H), 7.20(d, J = 2.4 Hz, 1H), 7.04 (d, J = 2.4 Hz, 1H), 6.71 (dd, J = 8.7, 2.4 Hz,1H), 3.76 (s, 3H), 3.72 (t, J = 7.6 Hz, 2H), 3.21 (t, J = 7.6 Hz, 2H); 13CNMR (150 MHz, DMSO-d6), δ (ppm): 153.6, 131.7, 127.5, 124.6, 112.6,111.8, 111.7, 100.4, 55.8, 34.9, 29.3.
References:
Li, Lin-Bo;Fan, Yong-Gang;Wu, Wen-Xi;Bai, Chen-Yang;Jia, Meng-Yu;Hu, Jiang-Ping;Gao, Hui-Ling;Wang, Tao;Zhong, Man-Li;Huang, Xue-Shi;Guo, Chuang [Bioorganic Chemistry,2022,vol. 128,art. no. 106100]
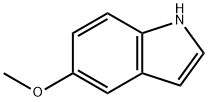
1006-94-6
504 suppliers
$10.00/5g

18334-96-8
9 suppliers
inquiry
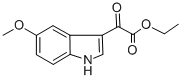
14771-33-6
38 suppliers
$49.47/250mgs:

18334-96-8
9 suppliers
inquiry
2426-19-9
6 suppliers
inquiry

18334-96-8
9 suppliers
inquiry