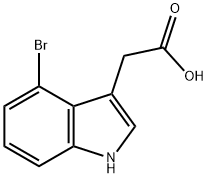
1H-Indole-3-aceticacid,4-bromo-(9CI) synthesis
- Product Name:1H-Indole-3-aceticacid,4-bromo-(9CI)
- CAS Number:89245-41-0
- Molecular formula:C10H8BrNO2
- Molecular Weight:254.08
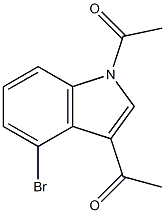
65018-18-0

89245-41-0
To a dry three-necked flask was added 98.0 kg of 4-bromoindole, 307.0 kg of aluminum trichloride and 500 L of methylene chloride, and 79.0 kg of acetyl chloride was added slowly and dropwise to the three-necked flask. The reaction mixture was heated to 40 °C and kept for 8 hours. Upon completion of the reaction, the mixture was poured into 800 L of ice water, the organic phase was separated and the solvent was recovered to give 1,3-diacetyl-4-bromoindole. Subsequently, 161 kg of morpholine and 40.0 kg of sulfur were added directly to the reaction system, heated to 120 °C and kept for 5 hours. After the rearrangement reaction was completed, 500 L of methanol was added, heated to dissolve, decolorized with activated carbon, filtered and cooled. To the filtrate, 300 L of 70% ethanol and 200 L of 15% sodium hydroxide solution were added and heated to reflux for 4 hours. After completion of the reaction, filter and recover the solvent under vacuum. To the residue, appropriate amount of water was added and the pH was adjusted to 1-2 with dilute hydrochloric acid to precipitate a solid. It was filtered, washed with water to neutral, and finally recrystallized with 60% ethanol to obtain 102.2 kg of 4-bromoindole-3-acetic acid in 85.2% yield.

65018-18-0
0 suppliers
inquiry

89245-41-0
62 suppliers
$49.00/100mg
Yield: 85.2%
Reaction Conditions:
Stage #1:1,3-diacetyl-4-bromoindole with morpholine;sulfur at 120; for 5 h;Industrial scale;Willgerodt-Kindler Rearrangement;
Stage #2: with sodium hydroxide in ethanol for 4 h;Reflux;Industrial scale;
Steps:
6 Synthesis of 4-bromoindole-3-acetic acid
To a dry three-necked flask was added 98.0 kg of 4-bromoindole, 307.0 kg of aluminum trichloride, 500 L of dichloromethane, 79.0 kg of acetyl chloride was slowly added dropwise to a three-necked flask and heated to 40 ° C for 8 h. The mixture was poured into 800 L of ice water, the organic phase was separated and the solvent was recovered to give 1,3-diacetyl-4-bromoindole. 16 lkg of morpholine and 40.0 kg of sulfur were directly added and heated to 120 ° C for 5 h. After the rearrangement reaction was completed, 500 L of methanol was added, dissolved by heating, decolorized by activated charcoal, filtered, cooled, added 300L 70% ethanol and 200L 15% sodium hydroxide solution, and the mixture was heated under reflux for 4 hours. Reaction completed, filtration, vacuum recovery solvent, add appropriate amount of water, diluted with dilute hydrochloric acid ρH- = 1 ~ 2, precipitation of solid. Filtered, washed with water to neutral, and recrystallized from 60% ethanol to give 102.2 kg 4-bromoindole-3-acetic acid in 85.2% yield.
References:
Southeast University;GE, YUHUA;PAN, DONGHUI;KANG, JINGYI CN104311469, 2016, B Location in patent:Paragraph 0070-0072
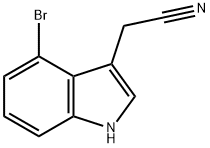
89245-35-2
44 suppliers
inquiry

89245-41-0
62 suppliers
$49.00/100mg

89434-01-5
0 suppliers
inquiry

89245-41-0
62 suppliers
$49.00/100mg
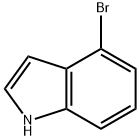
52488-36-5
469 suppliers
$15.00/1g

89245-41-0
62 suppliers
$49.00/100mg
4769-97-5
350 suppliers
$6.00/250mg

89245-41-0
62 suppliers
$49.00/100mg