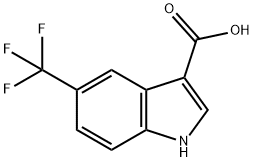
1H-INDOLE-3-CARBOXYLIC ACID,5-(TRIFLUOROMETHYL)- synthesis
- Product Name:1H-INDOLE-3-CARBOXYLIC ACID,5-(TRIFLUOROMETHYL)-
- CAS Number:948579-72-4
- Molecular formula:C10H6F3NO2
- Molecular Weight:229.16
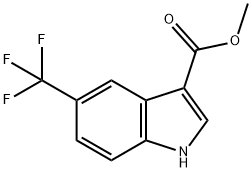
415918-11-5
3 suppliers
inquiry

948579-72-4
20 suppliers
inquiry
Yield:948579-72-4 100%
Reaction Conditions:
with sodium chlorite;sodium dihydrogenphosphate in tetrahydrofuran;water;tert-butyl alcohol at 20; for 24 h;
Steps:
168 Preparation 168: 5-(trifluoromethyl)-1H-indole-3-carboxylic acid
In a 100 mL round-bottomed flask, 200 mg of 5-(trifluoromethyl)-1H-indole-3-carbaldehyde (preparation 165; 94 mmol; 1 eq) were dissolved to 20 mL of tert-butanol 4.7 mL of 2M 2-methyl-2-butene in THF (9.38 mmol; 10 eq) were added. 764 mg of NaClO2 (8.44 mmol; 9 eq) and 1024 mg of Na H2PO4 2H2O (6.57 mmol; 7 eq) were dissolved in 8 mL of water and were added dropwise. The reaction medium was left to stir at RT for 24 h. The reaction medium was concentrated under reduced pressure and was then taken up in water and acidified to pH=3 with a 1N HCl solution. The aqueous phase was extracted 3 times with EtOAc and the organic phases were combined, dried over MgSO4, filtered and concentrated under reduced pressure. 247 mg of the title compound were obtained in the form of an orange solid.Yld: quantitative.1H NMR (300 MHz, CMCl3-d) δppm 7.95 ( d, J=3.1 Hz, 1H) 8.08 (d, J=3.1 Hz, 1H) 8.53 (s, 1H) 8.66 (s, 1H) 8.93 (brs, 1H) 10.10 (s, 1H)LC-MS: m/z (M+H)+: 228.
References:
US2017/66717,2017,A1 Location in patent:Paragraph 1454-1457