
1H-Indole-3-ethanamine, 6-fluoro-α-methyl- synthesis
- Product Name:1H-Indole-3-ethanamine, 6-fluoro-α-methyl-
- CAS Number:712-11-8
- Molecular formula:C11H13FN2
- Molecular Weight:192.24

720-13-8
0 suppliers
inquiry

712-11-8
2 suppliers
inquiry
Yield:-
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 3.5 h;
Steps:
8.B EXAMPLE 8; PREPARATION OF N-PROPYL 3-(4-FLUOROBENZOYL)-2-METHYL-8-FLUORO-1,2,3,6-TETRAHYDROAZEPINO[4,5-B]INDOLE-5-CARBOXYLATE
B. α-Methyl-6-fluorotrptamine was prepared by the dropwise addition of an anhydrous THF solution of 6-fluoro-3-(2-nitropropenyl)-1H-indole (560 mg, 2.54 mmol) to a stirred mixture of LiAIH4 (480 mg, 12. 7 mmol) in THF (40 mL) at 0 C. The reaction slurry was allowed to stir under N2 from 0 C to ambient room temperature over 3.5 hours. The reaction solution was again cooled to 0 C prior to the dropwise addition of H20 to quench excess LiAIH4. The reaction suspension was filtered through a pad of Celite to remove Al salts. The filtrate was concentrated under reduced pressure, diluted with EtOAc (200 mL), washed with NaCI (50 mL x 2), dried over Na2SO4, filtered and concentrated under reduced pressure to provide 450 mg of a-methyl-6-fluorotryptamine as a viscous oil ;'H NMR (CDCl3) 8 8.30 (1 H, br s), 7.52 (1H, m), 6.98-7. 04 (2H, m), 6.87 (1H, t), 3.29 (1H, m), 2.87 (1H, dd), 2.64 (1H, q), 1.25 (3H, d); MS (ESI) : 193 (MH+) ; TLC (SiO2 plate, 100 % DCM) Rf= 0.1.
References:
WO2003/99821,2003,A1 Location in patent:Page 104
![1H-Indole, 6-fluoro-3-[(1E)-2-nitro-1-propen-1-yl]-](/CAS/20210305/GIF/668420-25-5.gif)
668420-25-5
0 suppliers
inquiry

712-11-8
2 suppliers
inquiry
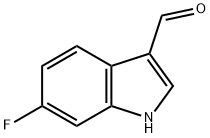
2795-41-7
169 suppliers
$7.00/250mg

712-11-8
2 suppliers
inquiry