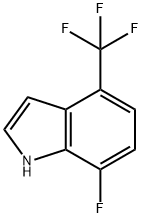
1H-Indole, 7-fluoro-4-(trifluoroMethyl)- synthesis
- Product Name:1H-Indole, 7-fluoro-4-(trifluoroMethyl)-
- CAS Number:1167055-51-7
- Molecular formula:C9H5F4N
- Molecular Weight:203.14
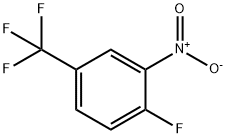
367-86-2
248 suppliers
$5.00/5g
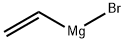
1826-67-1
211 suppliers
$60.89/100ml

1167055-51-7
11 suppliers
inquiry
Yield:1167055-51-7 19%
Reaction Conditions:
Stage #1: 1-fluoro-2-nitro-4-trifluoromethyl-benzene;vinyl magnesium bromide in tetrahydrofuran at -78; for 1 h;
Stage #2: with ammonium chloride in tetrahydrofuran;
Steps:
13.A
A. 7-Fluoro-4-trifluoromethyl-1 /-/-indole To a mixture of 1-fluoro-2-nitro-4-trifluoromethyl-benzene (3.27 ml_, 23.3 mmol) in THF (100 ml_) at -78 0C is added vinylmagnesium bromide (100 ml_, 0.7 M in THF, 70 mmol) slowly over a 30 min period. After this mixture is stirred at -78 °C for 30 min, sat. NH4CI is added, and the cooling bath is removed. The reaction mixture is partitioned between H2O and EtOAc, and the two layers are separated. The organic layer is washed with H2O and brine, dried over MgSO4, filtered, and concentrated in vacuo. The crude material is purified on silica gel with heptane/EtOAc (100/0 to 50/50) as eluant to give 0.93 g (19%) of the product as a brown oil. 1H NMR (CDCI3): δ 8.53 (br, s, 1 H), 7.50-7.15 (m, 2 H), 7.00-6.85 (m, 1 H), 6.85-6.70 (m, 1 H); 19 F NMR (CDCI3): δ -60.56 (s, 3F), -129.63 (d, J = 11.3 Hz, 1 F); MS 203 (M+, 100%).
References:
WO2011/22449,2011,A1 Location in patent:Page/Page column 78-79