
1H-Pyrazol-3-amine,5-(4-ethoxyphenyl)-(9CI) synthesis
- Product Name:1H-Pyrazol-3-amine,5-(4-ethoxyphenyl)-(9CI)
- CAS Number:129117-13-1
- Molecular formula:C11H13N3O
- Molecular Weight:203.24
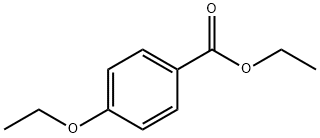
23676-09-7
160 suppliers
$5.00/1g
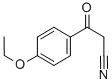
54605-62-8
35 suppliers
inquiry

129117-13-1
26 suppliers
$45.00/50mg
Yield:-
Reaction Conditions:
with hydrazine in tetrahydrofuran;n-butyllithium;ethanol;acetonitrile;
Steps:
38 5-(4-Ethoxy-phenyl)-2H-pyrazol-3-ylamine
5-(4-Ethoxy-phenyl)-2H-pyrazol-3-ylamine The named compound is the 3-aminopyrazole used in the reaction of Example 38 and can be prepared from 4-ethoxy-benzoic acid ethyl ester by the following method: Under Ar(g), a solution of anhydrous acetonitrile (1.5 mL) in 35 mL anhydrous THF is cooled to -78° C. in a dry ice-acetone bath and then slowly treated with 11 mL of a 2.5 M n-butyl lithium in hexanes. The reaction mixture is maintained at -78° C. for an additional 45 min. and then slowly treated with a solution of 5 gms. of 4-ethoxy-benzoic acid ethyl ester in 5 mL of anhydrous THF. The resulting solution is then stirred overnight at room temperature. Subsequently, the reaction mixture is treated with 10% NaOH(aq) to dissolve solids and then extracted with ethyl acetate. The aqueous layer is acidified (pH 6) with HCl(aq) affording a white precipitate which subsequently is filtered. The organic layer is concentrated in vacuo and the residue is triturated with isopropanol resulting in the formation of additional white precipitate. The combined precipitates provide the desired 3-(4-ethoxy-phenyl)-3-oxo-propionitrile in the amount of 2.5463 gms. 3-(4-Ethoxy-phenyl)-3-oxo-propionitrile (2.5463 gms.) is then suspended in 55 mL of anhydrous EtOH. This suspension is treated with 0.85 mL of anhydrous hydrazine and subsequently heated to refluxing temperature for 1 day. The reaction mixture is concentrated in vacuo. The residue is then triturated with isopropanol to precipitate out the 5-(4-ethoxy-phenyl)-2H-pyrazol-3-ylamine.
References:
US6559173,2003,B1
4640-67-9
301 suppliers
$9.00/1g

129117-13-1
26 suppliers
$45.00/50mg
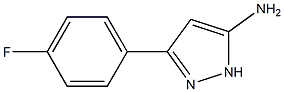
1242422-48-5
5 suppliers
inquiry

54605-62-8
35 suppliers
inquiry

129117-13-1
26 suppliers
$45.00/50mg

23676-09-7
160 suppliers
$5.00/1g

129117-13-1
26 suppliers
$45.00/50mg