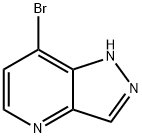
1H-Pyrazolo[4,3-b]pyridine, 7-broMo- synthesis
- Product Name:1H-Pyrazolo[4,3-b]pyridine, 7-broMo-
- CAS Number:1256806-33-3
- Molecular formula:C6H4BrN3
- Molecular Weight:198.02
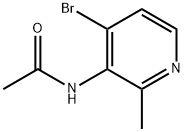
1350648-86-0
5 suppliers
inquiry
![1H-Pyrazolo[4,3-b]pyridine, 7-broMo-](/CAS/GIF/1256806-33-3.gif)
1256806-33-3
39 suppliers
$45.00/10mg
Yield: 17.35%
Reaction Conditions:
Stage #1:N-(4-bromo-2-methylpyridin-3-yl)acetamide with potassium acetate;acetic anhydride in toluene for 0.25 h;
Stage #2: with isopentyl nitrite in toluene at 85; for 4 h;
Steps:
48
[0226] To a solution of N-(4-bromo-2-methylpyridin-3-yl)acetamide (0.6 g, 2.62 mmol) in toluene (26.2 ml) was added potassium acetate (0.308 g, 3.14 mmol) and acetic anhydride (0.743 ml, 7.86 mmol). The mixture was stirred for 15 minutes and isopentyl nitrite (0.806 ml, 6.02 mmol) was added and the reaction was heated at 85°C for 4 hours. The reaction was cooled and partitioned between ethyl acetate (75 mL) and water (75 mL). The organic layer was separated, dried over MgS04, and concentrated to a brown oil. The oil was purified on silica column chromatography eluted with methanol :dichloromethane (3:97). Concentration of product fractions afforded 7-bromo-lH-pyrazolo[4,3-b]pyridine (90 mg, 0.454 mmol, 17.35 % yield) as an amber solid. 1H NMR (400 MHz, DMSO-d6) δ ppm 7.71 (1 H, d, J=4.80 Hz) 8.32 - 8.40 (1 H, m) 8.40 - 8.51 (1 H, m) 13.89 (1 H, br. s.). MS [M+H] found 197.9.
References:
TAKEDA PHARMACEUTICAL COMPANY LIMITED;KWOK, Lily;LARSON, John David;SABAT, Mark WO2011/146287, 2011, A1 Location in patent:Page/Page column 61
18614-66-9
31 suppliers
inquiry
![1H-Pyrazolo[4,3-b]pyridine, 7-broMo-](/CAS/GIF/1256806-33-3.gif)
1256806-33-3
39 suppliers
$45.00/10mg
18437-58-6
326 suppliers
$6.00/1g
![1H-Pyrazolo[4,3-b]pyridine, 7-broMo-](/CAS/GIF/1256806-33-3.gif)
1256806-33-3
39 suppliers
$45.00/10mg
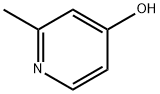
18615-86-6
147 suppliers
$21.00/250mg
![1H-Pyrazolo[4,3-b]pyridine, 7-broMo-](/CAS/GIF/1256806-33-3.gif)
1256806-33-3
39 suppliers
$45.00/10mg
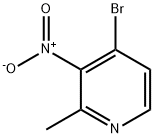
23056-49-7
19 suppliers
inquiry
![1H-Pyrazolo[4,3-b]pyridine, 7-broMo-](/CAS/GIF/1256806-33-3.gif)
1256806-33-3
39 suppliers
$45.00/10mg