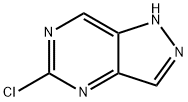
1H-Pyrazolo[4,3-d]pyriMidine, 5-chloro- synthesis
- Product Name:1H-Pyrazolo[4,3-d]pyriMidine, 5-chloro-
- CAS Number:633328-98-0
- Molecular formula:C5H3ClN4
- Molecular Weight:154.56
![Ethanone, 1-(5-chloro-1H-pyrazolo[4,3-d]pyriMidin-1-yl)-](/CAS/20150408/GIF/633328-97-9.gif)
633328-97-9
![1H-Pyrazolo[4,3-d]pyriMidine, 5-chloro-](/CAS/GIF/633328-98-0.gif)
633328-98-0
General procedure for the synthesis of 5-chloro-1H-pyrazolo[4,3-d]pyrimidin-1-yl from 1-(5-chloro-1H-pyrazolo[4,3-d]pyrimidin-1-yl)ethanone: To a stirred solution of 1-(5-chloro-1H-pyrazolo[4,3-d]pyrimidin-1-yl)ethanone (1.5 g, 7.65 mmol) in tetrahydrofuran (THF, 15 mL), was added an 8% hydrochloric acid aqueous solution (14.46 mL). The reaction mixture was refluxed at 50 °C for 30 min. After completion of the reaction, it was cooled to 25 °C and extracted with ethyl acetate (EtOAc, 2 × 50 mL). The organic layers were combined, dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure to afford 5-chloro-1H-pyrazolo[4,3-d]pyrimidine as an off-white solid (1.1 g, 93% yield). Mass spectrum (ESI): m/z = 153.0 [M-H]-.
![Ethanone, 1-(5-chloro-1H-pyrazolo[4,3-d]pyriMidin-1-yl)-](/CAS/20150408/GIF/633328-97-9.gif)
633328-97-9
20 suppliers
$462.00/1g
![1H-Pyrazolo[4,3-d]pyriMidine, 5-chloro-](/CAS/GIF/633328-98-0.gif)
633328-98-0
83 suppliers
$69.00/100mg
Yield:633328-98-0 93%
Reaction Conditions:
with hydrogenchloride in tetrahydrofuran;water at 50; for 0.5 h;Reflux;
Steps:
c) 5-Chloro-1H-pyrazolo[4,3-d]pyrimidine
To a stirred solution of 1-(5-chloro-pyrazolo[4,3-d]pyrimidin-1-yl)-ethanone (1.5 g, 7.65 mmol) in THF (15 mL) was added 8% aqueous HC1 (14.46 mL) at 50°C and reaction mixture was refluxed for 30 mm. After completion of the reaction, it was cooled to 25°C and was extractedwith EtOAc (2xSOmL). The combined organic layers were dried over Na2504, filtered and concentrated under reduced pressure to yield the title compound as an off-white solid (1.1 g, 93%). MS (ESI): mlz = 153.0 [M-H].
References:
F. HOFFMANN-LA ROCHE AG;HOFFMANN-LA ROCHE INC.;BUETTELMANN, Bernd;KOCER, Buelent;KUHN, Bernd;PRUNOTTO, Marco;RICHTER, Hans;RITTER, Martin WO2017/137334, 2017, A1 Location in patent:Page/Page column 123; 124
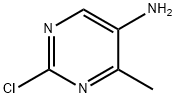
20090-69-1
114 suppliers
inquiry
![1H-Pyrazolo[4,3-d]pyriMidine, 5-chloro-](/CAS/GIF/633328-98-0.gif)
633328-98-0
83 suppliers
$69.00/100mg
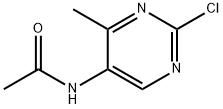
633328-96-8
17 suppliers
$220.00/50mg
![1H-Pyrazolo[4,3-d]pyriMidine, 5-chloro-](/CAS/GIF/633328-98-0.gif)
633328-98-0
83 suppliers
$69.00/100mg
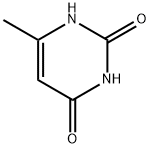
626-48-2
393 suppliers
$10.00/25 g
![1H-Pyrazolo[4,3-d]pyriMidine, 5-chloro-](/CAS/GIF/633328-98-0.gif)
633328-98-0
83 suppliers
$69.00/100mg
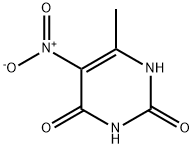
16632-21-6
245 suppliers
$5.00/1g
![1H-Pyrazolo[4,3-d]pyriMidine, 5-chloro-](/CAS/GIF/633328-98-0.gif)
633328-98-0
83 suppliers
$69.00/100mg