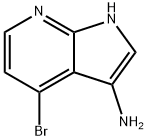
1H-Pyrrolo[2,3-b]pyridin-3-amine, 4-bromo- synthesis
- Product Name:1H-Pyrrolo[2,3-b]pyridin-3-amine, 4-bromo-
- CAS Number:943323-65-7
- Molecular formula:C7H6BrN3
- Molecular Weight:212.05
![1H-Pyrrolo[2,3-b]pyridine, 4-bromo-3-nitro-](/CAS/GIF/943323-63-5.gif)
943323-63-5
![1H-Pyrrolo[2,3-b]pyridin-3-amine, 4-bromo-](/CAS/GIF/943323-65-7.gif)
943323-65-7
4-Bromo-3-nitro-1H-pyrrolo[2,3-b]pyridine (500 mg, 2.07 mmol) was used as starting material, which was dissolved in 48% aqueous hydrobromic acid (4 mL) and heated at 70 °C. Subsequently, tin(II) chloride dihydrate (2.26 g, 10 mmol) was added in batches. After the addition was completed, the reaction was continued at 70 °C for 1 hour. Upon completion of the reaction, the mixture was cooled and carefully poured into a stirred ice-water mixture (15 mL). The pH of the reaction mixture was adjusted to 12 with sodium hydroxide solution and the insoluble material was separated by filtration. The filtrate was extracted with dichloromethane (3 x 100 mL), the organic phases were combined, washed sequentially with water and saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The target product 4-bromo-1H-pyrrolo[2,3-b]pyridin-3-amine (230 mg, 52.5% yield) was obtained, which could be used in the subsequent reaction without further purification.
![1H-Pyrrolo[2,3-b]pyridine, 4-bromo-3-nitro-](/CAS/GIF/943323-63-5.gif)
943323-63-5
18 suppliers
inquiry
![1H-Pyrrolo[2,3-b]pyridin-3-amine, 4-bromo-](/CAS/GIF/943323-65-7.gif)
943323-65-7
37 suppliers
$225.00/0.25G
Yield:943323-65-7 52.5%
Reaction Conditions:
with tin(II) chloride dihdyrate;hydrogen bromide in water at 70; for 1 h;
Steps:
3.2 Step 2: Preparation of 4-Bromo-1 H-pyrrolo[2,3-b]pyridin-3-ylamine
4-Bromo-3-nitro-1H-pyrrolo[2,3-b]pyridine (500mg, 2.07mmol) was heated in 48% aqueous hydrobromic acid (4mL) at 70°C and then tin (II) chloride dihydrate(2.26g, 10mmol) was added in portions. After addition the reaction was heated at 70°C for a further 1 hour and then cooled before carefully adding to stirring ice/H20 (l5mL). This solution was basified to pH 12 using sodium hydroxidesolution, and the insoluble material separated via filtration. The filtrate wasextracted with DCM (3xlOOmL) and the combined extracts were washed with H2O and saturated aqueous sodium chloride, dried (Mg504) and concentrated in vacuo. This afforded the desired compound, 230mg, 52.5%, which was used without further purification.
References:
WO2013/114113,2013,A1 Location in patent:Page/Page column 26
348640-06-2
338 suppliers
$6.00/250mg
![1H-Pyrrolo[2,3-b]pyridin-3-amine, 4-bromo-](/CAS/GIF/943323-65-7.gif)
943323-65-7
37 suppliers
$225.00/0.25G