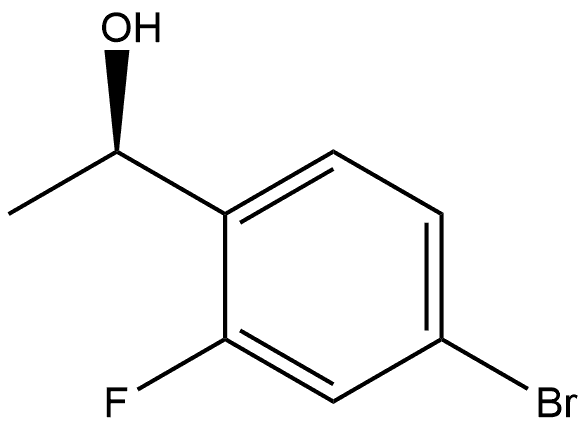
(1R)-1-(4-bromo-2-fluorophenyl)ethan-1-ol synthesis
- Product Name:(1R)-1-(4-bromo-2-fluorophenyl)ethan-1-ol
- CAS Number:749932-80-7
- Molecular formula:C8H8BrFO
- Molecular Weight:219.05
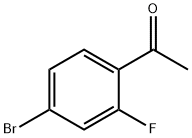
625446-22-2
195 suppliers
$6.00/250mg

749932-80-7
6 suppliers
inquiry
Yield:749932-80-7 90%
Reaction Conditions:
Stage #1: with dimethylamine borane;(S)-1-methyl-3,3-diphenyl-hexahydropyrrolo[1,2-c][1,3,2]oxazaborole in dichloromethane;toluene at -30; for 0.25 h;
Stage #2: 4-bromo-2-fluoroacetophenone in dichloromethane;toluene at 25;
Steps:
B.115.3 Step 3: (R) 1- (4-BROMO-2-FLUORO-PHENYL)-ETHANOL
An oven dried 500 mL flask, was charged under nitrogen with (S)-2-methyl-CBS- oxazaborolidine 1M in toluene (5.02 mL) and dissolved in CH2CI2 (250 mL). ME2-BH3 (30 mL, 60.27 mmol) was then added and cooled to-30 C and reaction stirred for 15 minutes. (1- (4- Bromo-2-fluoro-phenyl)-ethanone (10.9 g, 50.23 mmol) from step 2 below was dissolved in CH2CI2 (10 mL) and slowly added via addition funnel to the previous solution. The resulting reaction was stirred at 25 C overnight. The solution was carefully quenched with MeOH, the solvent was removed in vacuo and the residue was purified by flash column chromatography (20% EtOAc in hexanes) to provide the desired product (9 37 g, 90%) as a clear OIL. H NMR (400MHZ, CDCI3) : 5 1.49 (d, J = 6. 6 Hz, 3H), 5.15 (q, J = 12,6. 4Hz, 1 H), 7.15-7. 45 (m, 3H).
References:
WO2004/74270,2004,A2 Location in patent:Page 365-366