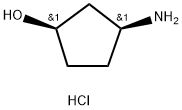
(1R,3S)-3-AMinocyclopentanol hydrochloride synthesis
- Product Name:(1R,3S)-3-AMinocyclopentanol hydrochloride
- CAS Number:1279032-31-3
- Molecular formula:C5H12ClNO
- Molecular Weight:137.61
![Carbamic acid, [(1S,3R)-3-hydroxycyclopentyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/167465-99-8.gif)
167465-99-8

1279032-31-3
Under nitrogen protection, 82 g of isopropanol was added to the reaction flask. Stirring was turned on, the reaction temperature was controlled at 5 °C, and 93.4 g of pivaloyl chloride was slowly added dropwise. After the dropwise addition, the reaction temperature was adjusted to 25 °C and the esterification reaction was carried out for 30 min. Subsequently, a solution prepared from 52 g of Compound III dissolved in 53 g of isopropanol was added dropwise to the reaction system. After completion of the dropwise addition, the deprotection reaction was carried out at room temperature for 12 h. The reaction process was monitored by GC. At the end of the reaction, the temperature of the reaction system was controlled at 0 °C and stirring was continued for 1 h to ensure complete precipitation of solids. Filtration was carried out under nitrogen protection and the resulting filter cake was washed with isopropanol at 5 °C until the washings were colorless. Next, the filter cake was washed with 40 g of acetone, stirred and heated to 50 °C and maintained for 2 hours. After completion of the reaction, the product was cooled to 0 °C and filtered under nitrogen protection, and the filter cake was washed with acetone at 5 °C until no droplets fell. Finally, the product was dried under vacuum at 40 °C for 12 h to give 25.4 g of (1R,3S)-3-aminocyclopentanol hydrochloride, which was 99.75% pure by GC, with less than 0.01% optical isomer impurities, and 69.8% overall yield based on Compound I.
![Carbamic acid, [(1S,3R)-3-hydroxycyclopentyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/167465-99-8.gif)
167465-99-8
114 suppliers
$35.00/100mg

1279032-31-3
169 suppliers
$39.00/100mg
Yield:1279032-31-3 95%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane at 20; for 2 h;
Steps:
4 Example 4:Compounds of formula V undergo a de Boc reaction to form a salt to give a compound of formula VI
10 g of the compound of formula V is dissolved in 20 mL of dioxane,Add 50 mL of dioxane hydrochloride (4M) and stir at room temperature for 2 h. Spin the reaction solution,100 mL of acetonitrile was beaten, suction filtered, and the filter cake was rinsed with 100 mL of acetonitrile.Dry blast to give 6.4 g of a white solid.The yield was 95%.
References:
Changzhou Pharmaceutical Factory Co., Ltd.;Sun Guangxiang;Zhang Yunran;Sun Haijiang;Wang Minfeng;Tao Weijie;Fu Jun;Ma Xuwei;Li Xingang CN109020911, 2018, A Location in patent:Paragraph 0085; 0086; 0087; 0088; 0089; 0090
1110772-05-8
96 suppliers
$25.00/10 mg

1279032-31-3
169 suppliers
$39.00/100mg
542-92-7
93 suppliers
inquiry

1279032-31-3
169 suppliers
$39.00/100mg
![Ethanone, 2-hydroxy-1-(1S,4R)-2-oxa-3-azabicyclo[2.2.1]hept-5-en-3-yl-2-phenyl-, (2R)-](/CAS/20200611/GIF/126924-22-9.gif)
126924-22-9
1 suppliers
inquiry

1279032-31-3
169 suppliers
$39.00/100mg