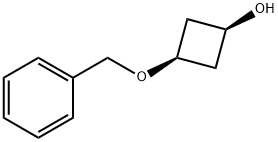
(1s,3s)-3-(benzyloxy)cyclobutan-1-ol synthesis
- Product Name:(1s,3s)-3-(benzyloxy)cyclobutan-1-ol
- CAS Number:233276-35-2
- Molecular formula:C11H14O2
- Molecular Weight:178.23
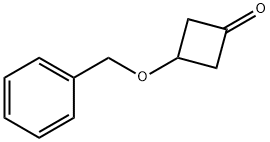
30830-27-4

233276-35-2
General procedure for the synthesis of cis-3-(benzyloxy)cyclobutanol from 3-(benzyloxy)-1-cyclobutanone: To a solution of 3-(benzyloxy)cyclobutan-1-one (300 mg, 1.71 mmol) in anhydrous ethanol (5 mL) was added sodium borohydride (65 mg, 1.71 mmol) in batches at 0 °C. The reaction mixture was stirred continuously at this temperature for 3 hours. Upon completion of the reaction, the reaction was quenched by the addition of ice water and extracted with ethyl acetate (EtOAc). The organic layers were combined, washed sequentially with water and saturated saline, dried over anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent. The resulting crude product was purified by silica gel column chromatography with hexane solution of 10% ethyl acetate as eluent to give cis-3-(benzyloxy)cyclobutanol as a light yellow oil (300 mg, 99% yield). The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 1.69-1.76 (m, 2H), 2.49-2.55 (m, 2H), 3.49-3.72 (m, 2H), 4.33 (s, 2H), 4.97-5.01 (m, 1H), 7.25-7.35 (m, 5H).

30830-27-4
217 suppliers
$27.00/1g

233276-35-2
53 suppliers
$106.00/100mg
Yield: 99%
Reaction Conditions:
with sodium tetrahydroborate;ethanol at 0; for 3 h;
Steps:
35 Preparation 35 Cis-3-(benzyloxy)cvclobutan-1-ol To a solution of 3-(benzyloxy)cyclobutan-1-one
Preparation 35 Cis-3-(benzyloxy)cvclobutan-1-ol To a solution of 3-(benzyloxy)cyclobutan-1-one (300 mg, 1.71 mmol) in EtOH (5 mL) at 0°C was added sodium borohydride (65 mg, 1.71 mmol) and the reaction was stirred at this temperature for 3 hours. The reaction was quenched by the addition of cold water and extracted into EtOAc. The organic layer was collected, washed with water, brine, dried over sodium sulphate and concentrated in vacuo. The residue was purified using silica gel column chromatography eluting with 10% EtOAc in hexanes to afford the title compound as a pale yellow oil (300 mg, 99%). 1 H NMR (400 MHz, DMSO-d6):5 ppm 1 .69-1.76 (m, 2H), 2.49-2.55 (m, 2H), 3.49-3.72 (m, 2H), 4.33 (s, 2H), 4.97-5.01 (m, 1 H), 7.25-7.35 (m, 5H).
References:
PFIZER INC.;BAGAL, Sharanjeet Kaur;OMOTO, Kiyoyuki;SKERRATT, Sarah Elizabeth;SWAIN, Nigel Alan;CUI, Jingrong Jean;MCALPINE, Indrawan James WO2016/9296, 2016, A1 Location in patent:Page/Page column 86-87
35995-55-2
23 suppliers
inquiry

233276-35-2
53 suppliers
$106.00/100mg

100-39-0
430 suppliers
$10.00/10 g

233276-35-2
53 suppliers
$106.00/100mg
100058-61-5
62 suppliers
$65.00/100mg

233276-35-2
53 suppliers
$106.00/100mg
![[[[3-(methylsulfinyl)-3-(methylthio)cyclobutyl]oxy]methyl]benzene](/CAS/20180703/GIF/917887-34-4.gif)
917887-34-4
5 suppliers
inquiry

233276-35-2
53 suppliers
$106.00/100mg