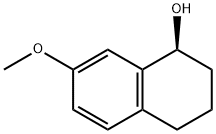
(1S)-7-methoxy-1,2,3,4-tetrahydronaphthalen-1-ol synthesis
- Product Name:(1S)-7-methoxy-1,2,3,4-tetrahydronaphthalen-1-ol
- CAS Number:182965-69-1
- Molecular formula:C11H14O2
- Molecular Weight:178.23
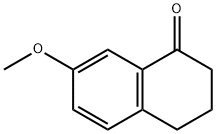
6836-19-7
499 suppliers
$14.00/5g

182965-69-1
9 suppliers
inquiry
Yield:182965-69-1 98%
Reaction Conditions:
with chloro{[(1S,2S)-(-)-2-amino-1,2-diphenylethyl](4-toluenesulfonyl)amido}-(mesitylene)ruthenium(II);formic acid;triethylamine in dichloromethane at 0 - 20; for 72 h;Inert atmosphere;Schlenk technique;enantioselective reaction;
Steps:
Preparation of (S)-7-Methoxy-1,2,3,4-Tetrahydronaphthalen-1-ol (S23) by Enantioselective Transfer Hydrogenation.
Following a modification of a literature procedure, triethylamine (2.79 mL, 2.00 equiv) was added to a 25-mL Schlenk flask and cooled to 0 °C in an ice-water bath. Formic acid (1.89mL, 5.00 equiv) was added, dropwise over 15 min, to reduce fuming and premature decomposition of the formate. The mixture was removed from the ice bath and RuCl[(S,S)-TsDPEN](mesitylene) (62.2 mg, 1.0 mol %) was added, in one portion, against nitrogen flow. 7-methoxy-3,4-dihydronaphthalen-1(2H)-one (1.76 g, 10.0 mmol) was then added in the same manner. CH2Cl2 (2.0 mL, 5.0 M ketone) was added with a syringe, rinsing down any solid stuck to the walls of the flask. The reaction was deemed complete by TLC after 72 h and quenched by the addition of water (5 mL). The biphasic mixture was then poured into a 125-mL separatory funnel and diluted with 1 M HCl (20 mL) and EtOAc (20 mL). The layers were shaken and separated, and the organic phase was washed with 1 M HCl (20 mL) and water (20 mL). Combined aqueous phases were extracted with EtOAc (2x 20 mL), and combined organic phases were washed with water (20 mL), 10% w/v aq NaOH (20 mL), water (20 mL), and brine (20mL), dried over MgSO4 (8 g), filtered, and concentrated under reduced pressure by rotary evaporation (40 °C, 20 mm Hg). Purification by silica gel column chromatography (5 cm x 15 cm column), eluting with a gradient of hexanes/EtOAc, 9:1 to hexanes/EtOAc, 3:1 gave S23 (1.75 g, 98%) as a clear, colorless oil. Spectroscopic data were consistent with the literature.
References:
Gilbert, Bradley B.;Eey, Stanley T.-C.;Ryabchuk, Pavel;Garry, Olivia;Denmark, Scott E. [Tetrahedron,2019,vol. 75,# 31,p. 4086 - 4098] Location in patent:supporting information