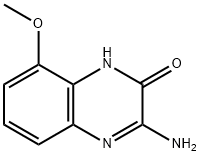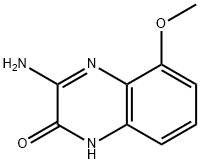
2(1H)-Quinoxalinone,3-amino-5-methoxy-(9CI) synthesis
- Product Name:2(1H)-Quinoxalinone,3-amino-5-methoxy-(9CI)
- CAS Number:659729-79-0
- Molecular formula:C9H9N3O2
- Molecular Weight:191.19
Yield:-
Reaction Conditions:
Stage #1: 3-methoxy-benzene-1,2-diamine hydrogen sulfatewith sodium hydrogencarbonate in ethanol;water;
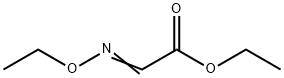
Stage #2: ethoxy-imino-acetic acid ethyl ester in ethanol;water at 20; for 16 h;
Steps:
7.c
(c) 3-Amino-8-methoxy-1H-quinoxalin-2-one and 3-amino-5-methoxy-1H-quinoxalin-2-one. To a suspension of 3-methoxy-benzene-1,2-diamine sulfate, Example 7(b), (2.36 g, 10 mmol) in EtOH (15 mL) and H2O (1 mL) was added NaHCO3 (1.68 g, 20 mmol, J T Baker). When gas evolution was complete, ethoxy-imino-acetic acid ethyl ester (1.6 g, 11 mmol, prepared according to J. Chem. Soc. Perkin. Trans. 1, 1999, 1789) was added and the mixture was stirred at room temperature for 16 h. The reaction was diluted with sat. aq. NaHCO3 and extracted with 25% i-PrOH/CHCl3 (5×). The combined organic layers were dried over Na2SO4, filtered, and concentrated in vacuo. Purification by flash chromatography (gradient: 0-5% MeOH/CH2Cl2) afforded 3-amino-8-methoxy-1H-quinoxalin-2-one as a light-brown powder [MS (ESI, pos. ion.) m/z: 192 (M+1)] and 3-amino-5-methoxy-1H-quinoxalin-2-one as a light-brown powder [MS (ESI, pos. ion.) m/z: 192 (M+1)].
References:
US2005/182067,2005,A1 Location in patent:Page/Page column 32