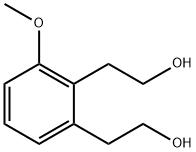
2,2'-(3-Methoxy-1,2-phenylene)diethanol synthesis
- Product Name:2,2'-(3-Methoxy-1,2-phenylene)diethanol
- CAS Number:847199-04-6
- Molecular formula:C11H16O3
- Molecular Weight:196.24
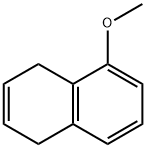
36230-47-4
10 suppliers
inquiry

847199-04-6
12 suppliers
$155.59/100mg
Yield:847199-04-6 89%
Reaction Conditions:
Stage #1: 1,4-dihydro-5-methoxynaphthalenewith ozone in ethanol;dichloromethane at -65; for 30 h;
Stage #2: with sodium tetrahydroborate in ethanol;dichloromethane at -65 - 20; for 0.5 h;
Steps:
Preparation 1
2,3-Bis-(2-hydroxyethviyi-methoxybenzene: ; Charge a four-neck 5 L flask equipped with an over-head mechanical stirrer, reflux condenser, thermocouple, and gas dispersion apparatus with 5-methoxy-l,4-dihydronaphthalene (264.54 g, 89.5% potency based on 1H-NMR, 1.478 mol) in DCM (1.3 L) and 2B-3 ethanol (1 L). Add sudan III (10 mg) to give a faint red color. Cool the solution to -65°C or lower, then pass O3 through the solution until the solution turns a light yellow color and the TLC (10:1 hexane/EtOAc, KMnO4 stain) shows the absence of the starting material (about 30 h). Transfer the solution via cannula into a slurry of NaBH4 (97.8 g, 2.59 mol) in 2B-3 ethanol (500 niL) cooled in ice/water. It is important that the temperature be maintained at or above O0C, as for example between O0C and 100C, throughout the transfer to ensure the ozonide is completely reduced to the diol. After the transfer is complete, warm the solution to ambient temperature and stir for about 30 min. Cool the slurry to 00C with ice/water then slowly add acetone (540 mL, 7.4 mol) to remove excess NaBH4. After all the solids dissolve, remove the solvent in vacuo. Dissolve the yellow solid in DCM (1 L) and water (1 L), separate the layers and extract the aqueous layer with DCM (750 mL). Wash the combined organic layers with brine (1.5 L), add toluene (750 mL) and remove the solvent in vacuo. Dissolve the solid in DCM (500 mL) with heating, then add toluene (750 mL) and concentrate the solution in vacuo to give the desired intermediate as a light yellow solid (283.7 g, 89% potency corrected, mp 82-83°C) (contains l,2,3,4-tetrahydro~5- methoxynaphthalene as an impurity (8.6%)). Further purify the product by vacuum drying overnight at 75°C, 5 Torr, to remove all but trace amount of the l,2,3,4-tetrahydro-5- methoxynaphthalene impurity. 1H NMR (300 MHz, CDCl3), δ 7.16 (dd, IH, / = 8.2,7.6), 6.83 (s, IH, J= 7.0), 6.76 (s, IH, J= 8.2), 3.85-3.77 (m, 7H), 3.01-2.91 (m, 4H), 2.35 (s, 2H); 13C NMR (300 MHz, DMSO-^6), δ 157.5, 138.9, 126.5, 125.2, 122.0, 108.4, 62.1, 60.5, 55.3, 36.1, 29.6; IR (KBr): 3006, 2960, 2886, 2829, 1583, 1461, 1440, 1264, 1091, 1041 cm4; MS (ES+) m/z 178 (M+H)+; Anal. Calc'd for C11H16O3: C, 67.32; H, 8.22; N, 0. Found: C, 67.26, H, 8.10, N, 0.21; Rf = 0.23 eluting with 95:5
References:
WO2007/28131,2007,A1 Location in patent:Page/Page column 36-42

36230-47-4
10 suppliers
inquiry

847199-04-6
12 suppliers
$155.59/100mg
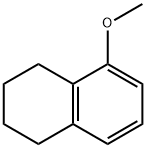
1008-19-1
38 suppliers
$155.00/100mg