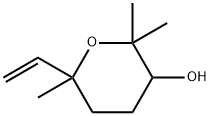
2,2,6-TRIMETHYL-6-VINYLTETRAHYDRO-2H-PYRAN-3-OL synthesis
- Product Name:2,2,6-TRIMETHYL-6-VINYLTETRAHYDRO-2H-PYRAN-3-OL
- CAS Number:14049-11-7
- Molecular formula:C10H18O2
- Molecular Weight:170.25
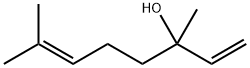
78-70-6
626 suppliers
$22.00/25mL
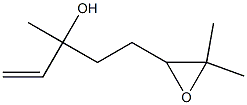
15249-35-1
2 suppliers
inquiry
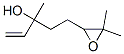
60047-17-8
109 suppliers
$49.00/5mL

14049-11-7
51 suppliers
$70.00/500mg
Yield:-
Reaction Conditions:
with [(n-C6H13)4N]3[PO4[WO(O2)2]4];dihydrogen peroxide in water;acetonitrile at 80; for 5 h;Catalytic behavior;
Steps:
The oxidation reactions of cis-cyclooctene (1), geraniol (2),linalool (3), linalyl acetate (4) and (-)-carveol (5) (Scheme 1) were carried out in acetonitrile, in a closed 5 mL reaction vessel equipped with a magnetic stirrer, using H2O2 (30 wt.%) as oxidant. The oxidation reactions of 1, 3, 4 and 5 were performed at 80∘C whereas the oxidation reactions of 2 were done at room temperature and protected from light. The homogeneous reactions were typically carried out as follows: the substrate and the required amount of catalyst were dissolved in acetonitrile (1.5 mL)and stirred; then the required amount of 30% (w/w) aqueous H2O2 was added to the reaction mixture. Aliquots were taken directly from the reaction mixture and injected into the GC-FID or GC-MS equipments for analysis of the starting materials and products. Similar conditions were used in heterogeneous reactions. The studiesin heterogeneous conditions were performed only with geraniol(2). The amount of catalyst used was 20 mg for BW4MIL-101 and40 mg for BW4aptesSiO2 and the aliquots were centrifuged andthen injected into the GC-FID or GC-MS equipments. At the endof the reactions the heterogeneous catalysts were separated from the reaction mixtures by centrifugation and washed with differentsolvents (CH3CN and C2H5OH) to remove the remaining substrate and oxidant, as well as the reaction products. The recovered catalysts were dried under vacuum at room temperature and used in a new reaction under identical experimental conditions, with readjustment of all quantities, without changing the molar ratios andreaction concentrations. The reaction products reported here wereidentified as described elsewhere [11,39,40]. In the case of linalooland (-)-carveol oxidation, the reaction products were identifiedby comparison to the GC-MS library. Blank reactions were performedfor all substrates, confirming that no oxidation productsare obtained unless the catalyst and H2O2 are both present. Even inthe presence of the supports (aptesSiO2 or MIL-101) and H2O2 nooxidation products were obtained. Acetamide was never observedamong the reaction products. Thus, the possibility of formation ofperoxyimidic acid, which would function as the oxidizing agent,was excluded.
References:
Santos, Isabel C.M.S.;Balula, Salete S.;Simões, Mário M.Q.;Cunha-Silva, Luís;Neves, M. Graça P.M.S.;De Castro, Baltazar;Cavaleiro, Ana M.V.;Cavaleiro, José A.S. [Catalysis Today,2013,vol. 203,p. 87 - 94]

78-70-6
626 suppliers
$22.00/25mL
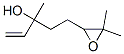
60047-17-8
109 suppliers
$49.00/5mL

14049-11-7
51 suppliers
$70.00/500mg

78-70-6
626 suppliers
$22.00/25mL
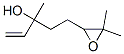
60047-17-8
109 suppliers
$49.00/5mL

14049-11-7
51 suppliers
$70.00/500mg
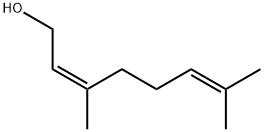
106-25-2
376 suppliers
$8.00/25g