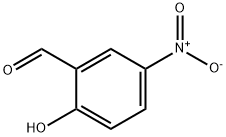
2-(2-chloroethoxy)-5-nitrobenzaldehyde synthesis
- Product Name:2-(2-chloroethoxy)-5-nitrobenzaldehyde
- CAS Number:110837-53-1
- Molecular formula:C9H8ClNO4
- Molecular Weight:229.62
Yield:110837-53-1 94%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 60;
Steps:
2-(2-Chloro-ethoxy)-5-nitro-benzaldehyde (XXId); (XXd) (xxid)To a mixture of (XXd) (1.Og, 5.98 mmol) and bromochloroethane (996 DL, 11.96 mmol) in dry DMF (15 mL) at ambient temperature was added potassium carbonate(1.64gg, 11.96mmol) and the resulting mixture was stirred at 6O0C overnight. The reaction mixture was cooled to O0C and quenched with H2O. The product was extracted with
References:
WO2007/58627,2007,A1 Location in patent:Page/Page column 77-78
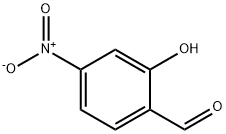
2460-58-4
135 suppliers
$32.00/250mg

107-06-2
441 suppliers
$14.00/25g

110837-53-1
22 suppliers
inquiry