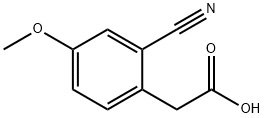
2-(2-cyano-4-Methoxyphenyl)acetic acid synthesis
- Product Name:2-(2-cyano-4-Methoxyphenyl)acetic acid
- CAS Number:52786-67-1
- Molecular formula:C10H9NO3
- Molecular Weight:191.18

1261620-25-0

52786-67-1
The general procedure for the synthesis of 2-(2-cyano-4-methoxyphenyl)acetic acid from the compound (CAS: 1261620-25-0) was as follows: methyl (2-cyano-4-methoxyphenyl)acetate (120 mg, 0.585 mmol) was dissolved in a solvent mixture of 10 mL of tetrahydrofuran (THF) and 2 mL of water, followed by addition of lithium hydroxide monohydrate ( LiOH-H2O, 101 mg, 2.34 mmol). The reaction mixture was heated and reacted at 55 °C overnight. After completion of the reaction, the mixture was cooled to room temperature and the solvent was removed under reduced pressure. The residue was dissolved in 30 mL of water and the pH was adjusted to 14 with 1 N aqueous sodium hydroxide (NaOH). the aqueous layer was washed with 50 mL of ethyl acetate and the organic phase was discarded. The aqueous phase was adjusted to pH 2-3 with 1 N hydrochloric acid (HCl) aqueous solution.Subsequently, the aqueous phase was extracted with 75 mL of ethyl acetate, the organic phases were combined and dried over anhydrous magnesium sulfate (MgSO4). After filtration, the organic phase was concentrated to afford 86 mg (77% yield) of the target product 2-(2-cyano-4-methoxyphenyl)acetic acid (SM-1ah) as a yellow solid. The mass spectrum (ESI+) showed the molecular ion peak m/z 192.2 ([M+H]+).1H NMR (CDCl3) δ 3.81 (s, 3H), 3.84 (s, 2H), 7.09 (dd, 1H), 7.13 (d, 1H), 7.30 (d, 1H), 10.22 (br s, 1H).

1261620-25-0
2 suppliers
inquiry

52786-67-1
35 suppliers
inquiry
Yield:52786-67-1 77%
Reaction Conditions:
Stage #1: (2-bromo-4-methoxy-phenyl)-acetic acidwith lithium hydroxide monohydrate;water in tetrahydrofuran at 55;
Stage #2: with water;sodium hydroxide pH=14;
Stage #3: with hydrogenchloride in water;ethyl acetate; pH=2 - 3;
Steps:
The (2-cyano-4-methoxy-phenyl)-acetic acid methyl ester (120 mg, 0.585 mmol) was dissolved in 10 mL THF and 2 mL water and LiOH monohydrate (101 mg, 2.34 mmol) was added. The mixture was heated at 55° C. overnight. The mixture was cooled to room temperature and the solvent was removed in vacuo. The residue was dissolved in 30 mL water and adjusted to pH 14 with aqueous 1N NaOH. The aqueous layer was washed with 50 mL of ethyl acetate. The organic extract was discarded and the aqueous phase was treated with 1N HCl (aq.) to pH2-3. The aqueous solution was washed with 75 mL ethyl acetate and the organic solution was dried (MgSO4), filtered and concentrated to give 86 mg (77%) of the title compound (SM-1ah) as a yellow solid. MS (ES+) 192.2 (M+H)+ 1H NMR (CDCl3) δ 3.81 (s, 3H), 3.84 (s, 2H), 7.09 (dd, 1H), 7.13 (d, 1H), 7.30 (d, 1H), 10.22 (br s, 1H).
References:
US2011/230461,2011,A1 Location in patent:Page/Page column 31
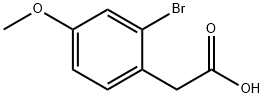
66916-99-2
131 suppliers
$11.00/1g

52786-67-1
35 suppliers
inquiry

198630-93-2
8 suppliers
inquiry

52786-67-1
35 suppliers
inquiry