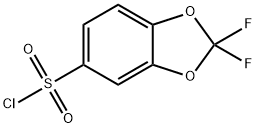
2,2-difluoro-1,3-benzodioxole-5-sulfonyl chloride synthesis
- Product Name:2,2-difluoro-1,3-benzodioxole-5-sulfonyl chloride
- CAS Number:313681-67-3
- Molecular formula:C7H3ClF2O4S
- Molecular Weight:256.61
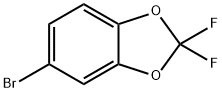
33070-32-5
195 suppliers
$6.00/1g

313681-67-3
7 suppliers
inquiry
Yield:313681-67-3 62%
Reaction Conditions:
Stage #1: 5-bromo-2,2-difluoro-2H-1,3-benzodioxolewith n-butyllithium in diethyl ether;hexane at -30; for 1.16667 h;
Stage #2: with sulfur dioxide in diethyl ether;hexane at -20; for 0.0833333 h;
Stage #3: with sulfuryl dichloride in hexane at 20; for 48 h;
Steps:
123.1
A solution of 2.37 g (10 mmol) 5-bromo-2,2-difluoro-1,3-benzodioxole in 25 ml anhydrous ether, cooled to -30° C., and 4 ml (10 mmol) n-butyllithium (2.5 M in hexanes) was added at -30° C., over 10 minutes. The mixture was stirred at -30° C. for 1 hour, then sulfur dioxide gas was passed through the mixture at -20° C. for 5 minutes. The mixture then was allowed to warm up to 20° C., and the precipitate was filtered and washed with 20 ml hexane. The solid was suspended in 20 ml hexanes, 1.35 g (10 mmol) sulfuryl chloride was added, the mixture was stirred at 20° C. for 48 hours. The mixture then was filtered and the filtrate was concentrated under reduced pressure to obtain 1.6 g (62%) title compound. The product was used in the next step without further purification. [0731] 1H-NMR (CDCl3): δ 7.27 (1H,d), 7.74 (1H,s), 7.88 (1H,d).
References:
US2004/122000,2004,A1 Location in patent:Page 129