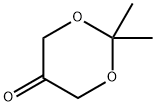
2,2-DIMETHYL-1,3-DIOXAN-5-ONE synthesis
- Product Name:2,2-DIMETHYL-1,3-DIOXAN-5-ONE
- CAS Number:74181-34-3
- Molecular formula:C6H10O3
- Molecular Weight:130.14
![(5-AMINO-2,2-DIMETHYL-[1,3]DIOXAN-5-YL)-METHANOL](/CAS/GIF/53104-32-8.gif)
53104-32-8

74181-34-3
The general procedure for the synthesis of 2,2-dimethyl-1,3-dioxan-5-one from 5-amino-2,2-dimethyl-1,3-dioxan-5-methanol was as follows: first, 2,2-dimethoxypropane (21.5 mL, 175 mmol) and p-toluenesulfonic acid monohydrate (1.58 g, 7.93 mmol) were added to a solution of tris(hydroxymethyl)aminomethane ( 25.2 g, 159 mmol) in a solution of N,N-dimethylformamide (DMF, 180 mL). The reaction mixture was stirred at room temperature for 22 hours. After completion of the reaction, the mixture was diluted with ethyl acetate and neutralized with the addition of triethylamine. Subsequently, the solvent was concentrated under reduced pressure to remove the solvent and the crude product obtained was purified by silica gel column chromatography (eluent: n-hexane/ethyl acetate, 5:1) to afford 2,2-dimethyl-5-amino-5-hydroxymethyl-1,3-dioxane (20.9 g, 78% yield) as a colorless solid. The structure of this intermediate was confirmed by 1H NMR (400 MHz, CDCl3): δ 3.80 (d, J = 12.0 Hz, 2H), 3.60 (d, J = 12.0 Hz, 2H), 3.53 (s, 2H), 2.61 (s, 2H), 1.45 (s, 3H), 1.41 (s, 3H). Next, the above intermediate (10.2 g, 62.1 mmol) was dissolved in water (60 mL) and potassium dihydrogen phosphate (KH2PO4, 8.51 g, 621 mmol) was added. An aqueous solution of sodium periodate (NaIO4) (0.5 M, 125 mL, 63 mmol) was slowly added dropwise at 0 °C. After completion of the dropwise addition, the reaction mixture was stirred for 1 h at room temperature. After completion of the reaction, the product was extracted with dichloromethane and the organic layer was washed sequentially with 5% aqueous sodium thiosulfate and saturated saline and dried over anhydrous sodium sulfate. Finally, the solvent was removed by concentration under reduced pressure to afford the target compound 2,2-dimethyl-1,3-dioxan-5-one (7.35 g, 91% yield) as a colorless oil. Its structure was confirmed by 1H NMR (400 MHz, CDCl3): δ 4.15 (s, 4H), 1.45 (s, 6H).
![(5-AMINO-2,2-DIMETHYL-[1,3]DIOXAN-5-YL)-METHANOL](/CAS/GIF/53104-32-8.gif)
53104-32-8
48 suppliers
$60.00/50mg

74181-34-3
135 suppliers
$13.00/100mg
Yield:74181-34-3 91%
Reaction Conditions:
with sodium periodate;potassium dihydrogenphosphate in water at 0 - 20; for 4 h;
Steps:
2.1 4.2.1. 2,2-Dimethyl-1,3-dioxane-5-one (8)
2,2-Dimethoxypropane (21.5 mL, 175 mmol) and p-toluenesulfonic acid monohydrate (1.58 g, 7.93 mmol) were added to a solution of tris(hydroxylmethyl)aminomethane (25.2 g, 159 mmol) inDMF (180 mL), and the mixture was stirred at room temperaturefor 22 h. The mixture was diluted with ethyl acetate, and then triethylamine was further added. The solvent was removed underreduced pressure, then the crude product was purified with silicagel column chromatography (eluent; n-hexane/ethyl acetate; 5:1)to give 2,2-dimethyl-5-amino-5-hydroxymethyl-1,3-dioxaone(20.9 g, 78%) as colorless solid. 2,2-Dimethyl-5-amino-5-hydroxymethyl-1,3-dioxaone: 1H NMR (400 MHz, CDCl3) d 3.80 (d,J = 12.0 Hz, 2H), 3.60 (d, J = 12.0 Hz, 2H), 3.53 (s, 2H), 2.61 (s, 2H),1.45 (s, 3H), 1.41 (s, 3H). The obtained intermediate (10.2 g,62.1 mmol) was dissolved in water (60 mL), and KH2PO4 (8.51 g,621 mmol) was added to the solution. Then aqueous solution ofNaIO4 (0.5 M, 125 mL, 63 mmol) was added dropwise during 3 hat 0 C and then stirred for 1 h at room temperature. The mixturewas extracted with dichloromethane and the organic layer waswashed with 5% aqueous sodium thiosulfate and brine and driedwith Na2SO4. The solvent was removed under reduced pressureto give compound 8 (7.35 g, 91%) as colorless oil. 1H NMR(400 MHz, CDCl3) d 4.15 (s, 4H), 1.45 (s, 6H).
References:
Fujii, Shinya;Sekine, Ryota;Kano, Atsushi;Masuno, Hiroyuki;Songkram, Chalermkiat;Kawachi, Emiko;Hirano, Tomoya;Tanatani, Aya;Kagechika, Hiroyuki [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 21,p. 5891 - 5901] Location in patent:supporting information
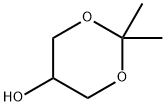
3391-30-8
31 suppliers
$250.00/500mg

74181-34-3
135 suppliers
$13.00/100mg
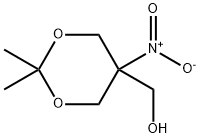
4728-14-7
13 suppliers
inquiry

74181-34-3
135 suppliers
$13.00/100mg
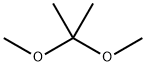
77-76-9
398 suppliers
$9.00/5ml

74181-34-3
135 suppliers
$13.00/100mg
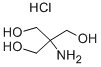
1185-53-1
610 suppliers
$7.00/25g

77-76-9
398 suppliers
$9.00/5ml

74181-34-3
135 suppliers
$13.00/100mg