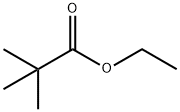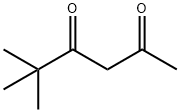
2,2-DIMETHYL-3,5-HEXANEDIONE synthesis
- Product Name:2,2-DIMETHYL-3,5-HEXANEDIONE
- CAS Number:7307-04-2
- Molecular formula:C8H14O2
- Molecular Weight:142.2
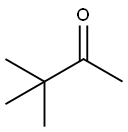
75-97-8

141-78-6

7307-04-2
The general procedure for the synthesis of 5,5-dimethylhexane 2,4-dione from pinacolone and ethyl acetate was as follows: pinacolone (2 g, 20 mmol) was dissolved in ether (2.4 mL) and slowly added dropwise to a stirred mixture of sodium hydride (0.96 g, 40 mmol, 60% oil solution) in anhydrous ethyl acetate (3.3 mL, 40 mmol), with the time of the addition being was controlled at 60 min to maintain a suitable reaction rate. The temperature was maintained at 40-50 °C by gentle heating in an oil bath during the reaction. If the reaction is too violent during the titration, the titration can be suspended and a small amount of ethanol can be added to ease the reaction. After complete addition of pinacolone, the reaction mixture was continued to be stirred at 40-50 °C. To maintain the fluidity of the reaction mixture, an additional 20 mL of ether was added. Upon completion of the reaction, the mixture was cooled to room temperature and diluted with sufficient amount of ether. Subsequently, ethanol was added and stirred for about 20 minutes to quench the unreacted sodium hydride. After cooling to room temperature again, the mixture was slowly neutralized by adding ice water and an appropriate amount of HCl, controlling the temperature to be below 20 °C. After neutralization is complete, stirring is continued until all solids are dissolved. The ether phase was separated and the aqueous phase was extracted with ether. The combined ether extracts were washed sequentially with NaHCO3 solution and water. After removal of the solvent under reduced pressure, the residue was purified by ball-to-ball distillation (60 °C, 10 mmHg) to afford the target product 5,5-dimethylhexane 2,4-dione as a colorless liquid (2.61 g, 54% yield).

75-97-8
245 suppliers
$10.00/1g

141-78-6
683 suppliers
$20.50/250ml

7307-04-2
67 suppliers
$38.00/5g
Yield: 54%
Reaction Conditions:
with sodium hydride in diethyl ether;mineral oil at 40 - 50;Inert atmosphere;
Steps:
Pivaloylacetone (1b)
The solution of pinacolone (2g, 20mmol) in ether (2.4mL) was added to a stirred mixture of sodium hydride (0.96g, 40mmol, 60% in oil) in dry ethyl acetate (3.3mL, 40mmol) during 60min, so to maintain convenient rate of reaction. The temperature was kept at 40-50°C by gentle warming in an oil bath. After the addition of few mL of ketone solution, the addition was stopped and little alcohol was added. After all the pinacolone was added, the mixture was stirred at 40-50°C. Additional 20mL of ether was added during this time to maintain a fluid reaction mixture. The reaction mixture was cooled to rt and sufficient ether was added. Most of the unreactive sodium hydride was destroyed by the addition of ethanol and stirring for about 20min. The mixture was then cooled to rt and a mixture of ice water and slight excess of dil. HCl was added keeping the temperature below 20°C during neutralization. Stirring was continued until all solid was dissolved. The ether phase was separated and aqueous phase was extracted with ether. The combined ether extract was washed with NaHCO3 solution then with water. The solvent was removed and residue was purified by bulb-to-bulb distillation (60°Cat 10mm Hg) to obtain 1b as colorless liquid (2.61g, 54%).
References:
Saha, Debajyoti;Ghosh, Raju;Dutta, Ranjan;Mandal, Achintya Kumar;Sarkar, Amitabha [Journal of Organometallic Chemistry,2015,vol. 776,p. 89 - 97]
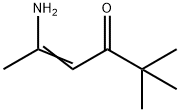
33663-62-6
0 suppliers
inquiry

7307-04-2
67 suppliers
$38.00/5g

75-97-8
245 suppliers
$10.00/1g

7307-04-2
67 suppliers
$38.00/5g