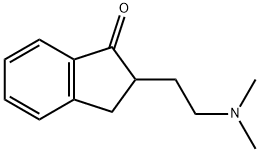
2-[2-(DiMethylaMino)ethyl]-1-indanone synthesis
- Product Name:2-[2-(DiMethylaMino)ethyl]-1-indanone
- CAS Number:3409-21-0
- Molecular formula:C13H17NO
- Molecular Weight:203.28
![1H-Inden-1-ol, 2-[2-(dimethylamino)ethyl]-2,3-dihydro-](/CAS/20210111/GIF/92196-07-1.gif)
92196-07-1
0 suppliers
inquiry
![2-[2-(DiMethylaMino)ethyl]-1-indanone](/CAS/GIF/3409-21-0.gif)
3409-21-0
45 suppliers
$139.80/10 mg
Yield: 79%
Reaction Conditions:
with benzophenone;potassium tert-butylate in benzene for 18 h;Heating / reflux;
Steps:
2
To a solution of LiAlH4 (7.8 g, 200 mmol) in THF (300 mL) at 0 °C, N, N- dimethyl-2- (1-oxo-indan-2-yl)-acetamide 1a (10 g, 46 mmol) in THF (40 mL) was added slowly. The reaction was allowed to stir at room temperature for half an hour and then at reflux for 6 hours. The reaction was cooled to room temperature and carefully quenched by the sequential addition of H20 (7.6 mL), 15% NaOH (7.6 mL) and H20 (22.8 mL). The mixture was stirred for a half hour, and the solid was filtered off using a celite pad and washed with excess THF. The combined filtrates were dried over Na2SO4 and concentrated to provide 2-(2-dimethylamino-ethyl)-indan- I -ol 2a as a yellow oil. A mixture of 2-(2- dimethylamino-ethyl)-indan-l-ol 2a, tBuOK (11.2 g, 100 mmol) and benzophenone (36.4 g, 200 mmol) in anhydrous benzene (200 mL) was refluxed for 18 hours under N2. The cooled mixture was poured into ice and extracted with 10% HCI until the HCI solution was colorless. The combined acid extracts were washed with Et20 (300 mL) and added dropwise with stirring into NH40H and ice. This basic solution was extracted thrice with Et20 (300 mL). The combined organic layers were washed with brine, dried over Na2S04, concentrated and purified by flash chromatography (CHCl3: MeOH:NH4OH, 95: 5:0.2) to give 2-(2-dimethylamino-ethyl)-indan- I -one 2b as a pale yellow oil in 79 % yield. (MH+= 204.2). The following compounds were also made according to this procedure: 2-(2-Dimethylamino-ethyl)-5-fluoro-indan-l-one 2c, 42% yield. (MH+ = 221.9) 2-(2-Dimethylamino-ethyl)-5-chloro-indan-l-one 2d, 81 % yield. (MH+ = 237.9) 2-(2-Dimethylamino-ethyl)-5-methyl-indan-l-one 2e, 62% yield. (MH+= 217.9) 2-(2-Dimethylamino-ethyl)-5-methoxy-indan-l-one 2f, 54% yield. (MH+ = 233.9)
References:
NEUROCRINE BIOSCIENCES, INC. WO2005/97093, 2005, A1 Location in patent:Page/Page column 32-33

867038-07-1
0 suppliers
inquiry
![2-[2-(DiMethylaMino)ethyl]-1-indanone](/CAS/GIF/3409-21-0.gif)
3409-21-0
45 suppliers
$139.80/10 mg
92501-97-8
6 suppliers
inquiry
![2-[2-(DiMethylaMino)ethyl]-1-indanone](/CAS/GIF/3409-21-0.gif)
3409-21-0
45 suppliers
$139.80/10 mg
-, diethyl ester (9CI)](/CAS/20200515/GIF/1805-03-4.gif)
1805-03-4
1 suppliers
inquiry
![2-[2-(DiMethylaMino)ethyl]-1-indanone](/CAS/GIF/3409-21-0.gif)
3409-21-0
45 suppliers
$139.80/10 mg
607-81-8
223 suppliers
$9.00/1g
![2-[2-(DiMethylaMino)ethyl]-1-indanone](/CAS/GIF/3409-21-0.gif)
3409-21-0
45 suppliers
$139.80/10 mg