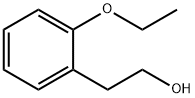
2-(2-ethoxyphenyl)ethanol synthesis
- Product Name:2-(2-ethoxyphenyl)ethanol
- CAS Number:22545-14-8
- Molecular formula:C10H14O2
- Molecular Weight:166.22
Yield:22545-14-8 65%
Reaction Conditions:
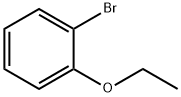
Stage #1: beta-bromophenetolewith iodine;magnesium in tetrahydrofuran at 0; for 0.5 h;Inert atmosphere;Reflux;
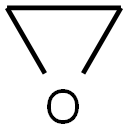
Stage #2: oxirane in tetrahydrofuran at 20;
Steps:
11 Synthesis of Compound 11.3.
Into a 250-mL 3-necked round-bottom flask, maintained with an inert atmosphere of nitrogen, was placed Mg (1.0 g, 41.67 mmol, 2.09 equiv) and I2 (10 mg).
Then a solution of 1-bromo-2-ethoxybenzene (4.0 g, 19.89 mmol, 1.00 equiv) in 25 mL THF was added dropwise and the resulting mixture was heated to reflux for 0.5 hr.
After the reaction was complete, the resulting mixture was cooled to 0° C. and then oxirane (50 mL) was added in one portion.
The reaction mixture was stirred overnight at room temperature.
The reaction was then quenched by the addition of 50 mL of NH4Cl (aq.) and extracted with 3*50 mL of ethyl acetate.
The organic layers were combined, dried over anhydrous sodium sulfate and concentrated under vacuum.
The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1:10).
Purification afforded 2.14 g (65%) of 2-(2-ethoxyphenyl)ethan-1-ol as a yellow oil.
Synthesis of Compound 11.4.
References:
US2013/123231,2013,A1 Location in patent:Paragraph 0399; 0402; 0403
7768-28-7
102 suppliers
$16.00/1g

75-03-6
358 suppliers
$11.00/5g

22545-14-8
11 suppliers
$473.00/5g
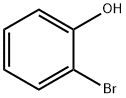
95-56-7
294 suppliers
$9.00/5G

22545-14-8
11 suppliers
$473.00/5g
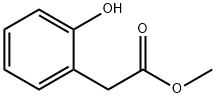
22446-37-3
109 suppliers
$8.00/250mg

22545-14-8
11 suppliers
$473.00/5g

41842-29-9
5 suppliers
inquiry

22545-14-8
11 suppliers
$473.00/5g