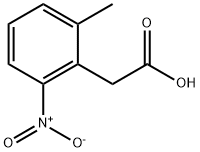
2-(2-Methyl-6-nitrophenyl)acetic acid synthesis
- Product Name:2-(2-Methyl-6-nitrophenyl)acetic acid
- CAS Number:23876-18-8
- Molecular formula:C9H9NO4
- Molecular Weight:195.17
83-41-0
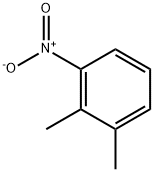
95-92-1

23876-18-8
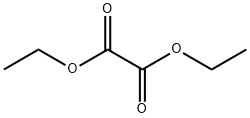
1. Diethyl oxalate (3.9 g, 27 mmol) was dissolved in 30 mL of diethyl ether and added slowly and dropwise to a 6 mL ether suspension of potassium ethanolate (2.3 g, 27 mmol) under stirring. 2. After refluxing had subsided, the reaction mixture was cooled in an ice bath, followed by the slow addition of 3-nitro-o xylene (2.8 g, 18 mmol) dissolved in 3 mL of diethyl ether while maintaining vigorous stirring. 3. The reaction mixture was refluxed for 15 minutes and a thick precipitate was observed to form. 4. The reaction mixture was heated under vacuum to remove most of the ether, followed by the slow addition of 18 mL of 10% sodium hydroxide solution with stirring. 5. hydrogen peroxide (4 mL, 30%) was added slowly and dropwise, during which the reaction was vigorous and accompanied by gas release. 6. Continue stirring the reaction mixture for 1.5 hours. 7. The solid product was collected by vacuum filtration, washed with water and discarded. 8. 8. The filtrate and washings were combined and acidified with 12 N hydrochloric acid to pH 2. 9. 9. The precipitated solid was again collected by vacuum filtration, washed with water and dried under vacuum. 10. The crude product was ground with dichloromethane and further dried under vacuum to give 1.8 g of 2-(2-methyl-6-nitrophenyl)acetic acid in 52% yield.
Yield:23876-18-8 52%
Reaction Conditions:
Stage #1: 2,3-dimethylnitrobenzene;oxalic acid diethyl esterwith potassium ethoxide in diethyl ether; for 0.25 h;Heating / reflux;
Stage #2: with sodium hydroxide;water;dihydrogen peroxide for 1.5 h;
Stage #3: with hydrogenchloride in water; pH=2;
Steps:
Diethyloxalate (3.9 g, 27 mmol) dissolved in 30 mL of diethyl ether was slowly added to a stirred mixture of 2.3 g (27 mmol) of potassium ethoxide in 6 mL of diethyl ether. When the refluxing subsided, the reaction was cooled in an ice bath and 2.8 g (18 mmol) of 3-nitro-o-xylene dissolved in 3 mL of diethyl ether was slowly added with vigorous stirring. The reaction was refluxed for 15 minutes resulting in a thick precipitate. The reaction was warmed under vacuum to remove most of the ether and 18 mL of 10% sodium hydroxide was slowly added with stirring. Hydrogen peroxide (4 mL of 30%) was slowly added accompanied by a vigorous reaction and evolution of gas. The mixture was stirred for 1.5 hours. The solids were collected by vacuum filtration, washed with water, and discarded. The filtrate and wash was combined and acidified to pH 2 with 12 N hydrochloric acid. The solids were collected by vacuum filtration, washed with water and dried under vacuum. The crude product was triturated with dichloromethane and dried under vacuum to give 1.8 g (52% yield) of 2-methyl-6-nitro-phenylacetic acid.
References:
US2004/186160,2004,A1 Location in patent:Page/Page column 22
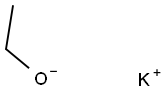
917-58-8
120 suppliers
$74.00/100g

95-92-1
439 suppliers
$5.00/5G
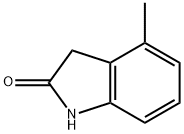
13220-46-7
92 suppliers
$16.00/250mg

23876-18-8
35 suppliers
$28.00/100mg

70467-01-5
0 suppliers
inquiry

23876-18-8
35 suppliers
$28.00/100mg

83-41-0
29 suppliers
$13.00/25g

23876-18-8
35 suppliers
$28.00/100mg