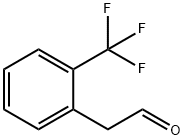
2-(2-(TRIFLUOROMETHYL)PHENYL)ACETALDEHYDE synthesis
- Product Name:2-(2-(TRIFLUOROMETHYL)PHENYL)ACETALDEHYDE
- CAS Number:21235-63-2
- Molecular formula:C9H7F3O
- Molecular Weight:188.15
94022-96-5
92 suppliers
$14.14/250mgs:

21235-63-2
15 suppliers
inquiry
Yield:21235-63-2 100%
Reaction Conditions:
with Silicycle in dichloromethane;
Steps:
187
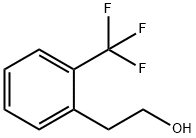
To a 40ml scintillation vial was added 0.067ml of 2-[(trifluoromethyl)phenyl]ethanol (0.42 mmoles, 1eq) and 5ml dichloromethane. Then 3g of 21 wt% pyridinium chlorochromate/silica gel (Silicycle, 21wt%, 4eq) was added and the reaction was shaken on an orbital shaker overnight. The reaction was filtered, washed with 3ml diethyl ether and concentrated at room temperature at 200 mBar until approximately 3ml of volume remained. The resulting aldehyde was neither isolated nor analyzed the crude product was used in the next step below. (100% yield was assumed.) To a 40ml scintillation vial was added 66mg 4-(tetrahydro-1(2H)-pyridazinyl)-1- naphthalenecarbonitrile (B1) (0.278 mmoles, 1eq), 3ml ethanol, 1ml acetic acid, and the above aldehyde solution, which contains a solution of the above aldehyde in 3ml dichloromethane (0.42mmoles, 1.5eq). Then 400mg (polystyrylmethyl)trimethylammoniumcyanoborohydride resin (Novabiochem, 4.3mmol/g, 5eq) was added and the reaction was shaken on an orbital shaker overnight. The reaction was filtered and concentrated in vacuo. The residue was purified via preparative HPLC (Phenomenex column Luna C18, 75mm x 30mm, 5 micron), 50% acetonitrile/water (0.01% TFA) to 100% acetonitrile, to yield 36mg of the title compound as a yellow oil.1H NMR (400 MHz, METHANOL-D4) δ ppm 1.8 (m, 4 H), 2.6 (t, J=7.2 Hz, 2 H), 3.1 (t, J=7.2 Hz, 2 H), 3.4 (m, 2 H), 3.4 (m, 2 H), 7.0 (m, 1 H), 7.0 (m, J=8.1 Hz, 1 H), 7.1 (m, 2 H), 7.4 (m, 1 H), 7.4 (m, 1 H), 7.6 (m, 1 H), 7.8 (d, J=7.9 Hz, 1 H), 8.0 (d, J=8.6 Hz, 1 H), 8.3 (d, J=8.8 Hz, 1 H)
References:
WO2006/91592,2006,A1 Location in patent:Page/Page column 126-127