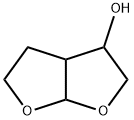
2,3,3a,4,5,6a-hexahydrofuro[2,3-b]furan-4-ol synthesis
- Product Name:2,3,3a,4,5,6a-hexahydrofuro[2,3-b]furan-4-ol
- CAS Number:109789-19-7
- Molecular formula:C6H10O3
- Molecular Weight:130.14
![tetrahydrofuro[2,3-b]furan-3(2H)-one](/CAS/20180713/GIF/445389-44-6.gif)
445389-44-6
6 suppliers
inquiry
![2,3,3a,4,5,6a-hexahydrofuro[2,3-b]furan-4-ol](/CAS/20180702/GIF/109789-19-7.gif)
109789-19-7
11 suppliers
inquiry
Yield:-
Reaction Conditions:
with methanol;sodium tetrahydroborate at -5 - 0; for 2 h;
Steps:
15
Example-15: Process for the enhancing the enantiomer of (3R, 3aS, 6aR)- Hexahydrofuro [2, 3-b] furan-3-ol.; Hexahydrofuro[2,3-b]furan-3-ol (100 g) was dissolved in dichloromethane (700ml). sodium bicarbonate (10% , 300 ml) was added to it and cooled to -5°C. Potassium bromide (3.7g), 2,2,6,6-Tetramethylpiperidin-1-yl)oxyl (TEMPO) (2.4g) and Tetrabutyl ammonium bromide (2.0g) was added to the reaction. Sodium hypochlorite solution (670 ml) was added slowly by maintaining temp 0 to -6°C. Reaction mass was maintained at 0 to -6°C for 1hr. Aqueous layer was separated and extracted with dichloromethane (2x200ml). All the dichloromethane layers were combined and washed with hydrochloride solution (10%, 200ml) and Sodium thiosulphate solution (10%, 200ml). Dichloromethane layer was distilled to half and then cooled to -5°C. Sodium borohydride (10.3g) was added to the reaction mass and then methanol (100ml) was added slowly drop-wise. Reaction mass was stirred for 2 hrs at 0 to -5°C, Acetic acid (16.1g) was added to the reaction mass and reaction mass was distilled out residue. Methanol (200ml) was added to it and stirred for 30min and again concentrated to residue. Residue was strip-off with ethyl acetate (100ml). Ethyl acetate (500ml) was added to the residue and cooled to 10-15°C. Reaction mass was filtered. Filtrate was concentrated to get residue of Hexahydrofuro [2,3-b] furan-3-ol (Yield 67g). 1H NMR : 1.81-1.91(m, 1H), 2.27-2.34(m, 1H), 2.82-2.90(m, 1 H), 3.61-3.67(m, 1 H), 3.86- 3.92(m, 1H), 4.41-4.48(m, 1H), 3.88-4.02 (m, 2H), 5.68 (d, 1H)
References:
MATRIX LABORATORIES LTD;VELLENKI, Siva Rama Prasad;SAHU, Arabinda;SHIMPI, Nitin Ashok;PONNURU, Anil Kumar;KOTHARI, Satish Babu WO2012/70057, 2012, A1 Location in patent:Page/Page column 19-20

1378929-91-9
0 suppliers
inquiry
![2,3,3a,4,5,6a-hexahydrofuro[2,3-b]furan-4-ol](/CAS/20180702/GIF/109789-19-7.gif)
109789-19-7
11 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![2,3,3a,4,5,6a-hexahydrofuro[2,3-b]furan-4-ol](/CAS/20180702/GIF/109789-19-7.gif)
109789-19-7
11 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![2,3,3a,4,5,6a-hexahydrofuro[2,3-b]furan-4-ol](/CAS/20180702/GIF/109789-19-7.gif)
109789-19-7
11 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![2,3,3a,4,5,6a-hexahydrofuro[2,3-b]furan-4-ol](/CAS/20180702/GIF/109789-19-7.gif)
109789-19-7
11 suppliers
inquiry