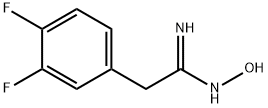
2-(3,4-DIFLUORO-PHENYL)-N-HYDROXY-ACETAMIDINE synthesis
- Product Name:2-(3,4-DIFLUORO-PHENYL)-N-HYDROXY-ACETAMIDINE
- CAS Number:200504-48-9
- Molecular formula:C8H8F2N2O
- Molecular Weight:186.16
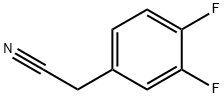
658-99-1
154 suppliers
$14.14/1gm:

200504-48-9
7 suppliers
inquiry
Yield:200504-48-9 89%
Reaction Conditions:
with hydroxyamino hydrochloride;anhydrous sodium carbonate in ethanol;water monomer at 80; for 3 h;
Steps:
Intermediate V-7; N-Hydroxy-2-phenyl-acetamidine
General procedure: Intermediate V-7; N-Hydroxy-2-phenyl-acetamidine To a solution of the commercially available phenyl-acetonitrile (1.0 g, 8.54 mmol) in ethanol (15 mL) and water (5.0 mL) were added Na2C03 (1.81 g, 17.1 mmol) and hydroxylamine hydrochloride (1.19 g, 17.1 mmol). The reaction mixture was heated to 80 °C for 4 hours, poured into 50 mL water and extracted with EtOAc (150 mL). The organic phase was dried over Na2S04 and concentrated under vacuo. The crude material was purified by flash chromatography (CE CVMeOH 95/5) to afford 1.16 g (90 %) of the title compound as a white solid. ES-MS m/e: 151.1 (M+H+).
References:
WO2015/140130,2015,A1 Location in patent:Page/Page column 143; 144