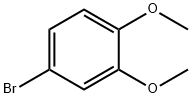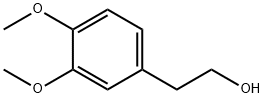
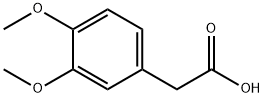
2-(3,4-Dimethoxyphenyl)ethanol synthesis
- Product Name:2-(3,4-Dimethoxyphenyl)ethanol
- CAS Number:7417-21-2
- Molecular formula:C10H14O3
- Molecular Weight:182.22
93-40-3

7417-21-2
General procedure for the synthesis of 3,4-dimethoxyphenylethanol from 3,4-dimethoxyphenylacetic acid: a dry and clean 100 mL round-bottomed flask was taken and sodium borohydride (0.4 g, 7.65 mmol) and iodine (1.3 g, 5.10 mmol) were accurately weighed and added to the flask, followed by the addition of 10 mL of tetrahydrofuran as solvent. The reaction vial was placed in an ice bath and stirred until homogeneous, then 3,4-dimethoxyphenylacetic acid (1.0 g, 5.10 mmol) was added slowly in 5 small portions. After the addition was completed, the ice bath was removed and the reaction was allowed to continue at room temperature. The progress of the reaction was monitored by thin layer chromatography (TLC). After about 30 minutes, TLC showed that the reaction was complete. To the reaction mixture, 50 mL of 5% sodium hydroxide solution was added and extracted with ethyl acetate (10 mL x 3), all organic phases were combined and washed with brine (10 mL x 3). The organic phase was separated, dried with anhydrous sodium sulfate, filtered and the solvent was evaporated to give 3,4-dimethoxybenzeneethanol (22) as a yellow oil in 98.9% yield.

93-40-3
472 suppliers
$10.00/5g

7417-21-2
233 suppliers
$29.00/5g
Yield:7417-21-2 98.9%
Reaction Conditions:
with sodium tetrahydroborate;iodine in tetrahydrofuran at 20; for 0.583333 h;Cooling with ice;Concentration;
Steps:
1.5 (5) Synthesis of Compound 22
Take a dry and clean 100 mL round bottom flask, accurately weigh sodium borohydride (0.4 g, 7.65 mmol) and iodine (1.3 g, 5.10 mmol) in a bottle, add 10 mL of tetrahydrofuran, place in an ice bath, stir After 5 min, 21 (1.0 g, 5.10 mmol) was added in small portions. After the addition, the ice bath was removed and the reaction was carried out at room temperature. The reaction was monitored by TLC. After 30 min, the reaction was completed by TLC. 50 ml of a 5% sodium hydroxide solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate (10 mL × 3), and the organic phase was combined and washed with brine (10 mL × 3). The organic phase was separated, dried over anhydrous sodium sulfate, filtered and evaporatedThe title compound (22) was obtained as a yellow oil.The yield was 98.9%.
References:
CN108484593,2018,A Location in patent:Paragraph 0050; 0051; 0065
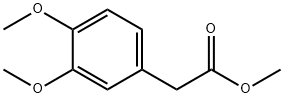
15964-79-1
105 suppliers
$10.00/1g

7417-21-2
233 suppliers
$29.00/5g
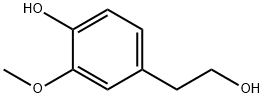
2380-78-1
122 suppliers
$68.00/250mg

74-88-4
357 suppliers
$15.00/10g

7417-21-2
233 suppliers
$29.00/5g
6380-23-0
91 suppliers
$30.00/1g

7417-21-2
233 suppliers
$29.00/5g