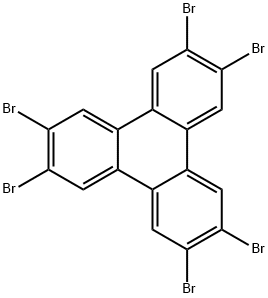
2,3,6,7,10,11-hexabromobenzo[9,10]phenanthrene synthesis
- Product Name:2,3,6,7,10,11-hexabromobenzo[9,10]phenanthrene
- CAS Number:82632-80-2
- Molecular formula:C18H6Br6
- Molecular Weight:701.66
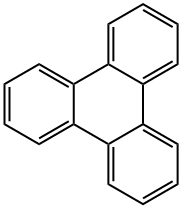
217-59-4
![2,3,6,7,10,11-hexabromobenzo[9,10]phenanthrene](/CAS/GIF/82632-80-2.gif)
82632-80-2
Bromine (9.0 mL, 349 mmol) was added dropwise to a solution of 9,10-benzophenanthrene (3.95 g, 17.3 mmol) and iron powder (410 mg, 7.34 mmol) in nitrobenzene (200 mL) under nitrogen protection with stirring at room temperature for 20 min, and the reaction was subsequently heated up to 210°C for 3 hours. After completion of the reaction, the mixture was cooled to room temperature and the product was precipitated by the addition of ether (Et2O). The precipitate was collected by filtration and washed thoroughly with ether, acetone and chloroform (CHCl3) sequentially to afford the white powdery product 2,3,6,7,10,11-hexabromobenzo[9,10]phenanthrene (11.5 g, 16.4 mmol, 94.8% yield). The melting point of the product was above 300°C; 1H NMR (300 MHz, CDCl3): δ = 8.72 (s, 6H, Ar-H); IR (KBr): νmax = 1585, 1551, 1454, 1365, 1119, 873, 863 cm-1.

217-59-4
306 suppliers
$5.00/250mg
![2,3,6,7,10,11-hexabromobenzo[9,10]phenanthrene](/CAS/GIF/82632-80-2.gif)
82632-80-2
119 suppliers
$45.00/50mg
Yield:82632-80-2 95%
Reaction Conditions:
with bromine;iron in nitrobenzene at 20 - 205; for 18.25 h;
Steps:
1.3 Example 1.3. Synthesis of 2,3,6,7,10,11-Hexabromotriphenylene (2)
To a solution of triphenylene (1, 1.07 g, 4.7 mmol) in nitrobenzene (40 mL) with iron powder (100 mg, 1.79 mmol) bromine (2.2 mL, 38.8 mmol) was slowly added over 15 minutes. The solution was then allowed to stand for 16 hours at room temperature. It was heated at 205 °C for 2 hours. The mixture was cooled to room temperature and mixed with diethyl ether (150 mL) and filtered. The crude white solid was washed by diethyl ether (3x30 mL) and acetone (3x10 mL). After drying in vacuo for 12 hours, 3.13 g of 2,3,6,7, 10,11-hexabromotriphenylene (yield 95%) was collected. The product was used directly without characterization due to low solubility.
References:
TRUSTEES OF DARTMOUTH COLLEGE;MIRICA, Katherine, A.;ZHAN, Xiaoping;MENDECKI, Lukasz;MENG, Zheng;KO, Michael WO2019/32804, 2019, A1 Location in patent:Paragraph 00173; 00174-00175
84-15-1
170 suppliers
$6.00/5g
![2,3,6,7,10,11-hexabromobenzo[9,10]phenanthrene](/CAS/GIF/82632-80-2.gif)
82632-80-2
119 suppliers
$45.00/50mg