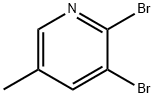
2,3-DIBROMO-5-METHYLPYRIDINE synthesis
- Product Name:2,3-DIBROMO-5-METHYLPYRIDINE
- CAS Number:29232-39-1
- Molecular formula:C6H5Br2N
- Molecular Weight:250.92
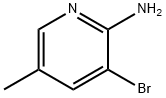
17282-00-7

29232-39-1
2-Amino-3-bromo-5-methylpyridine (187 mg, 1.00 mmol) was added to a 30 mL three-necked flask, followed by the addition of 48% aqueous hydrogen bromide (1 mL) to dissolve the feedstock. The reaction mixture was cooled in an ice bath, keeping the internal temperature in the range of 2 to 5 °C, and bromine (154 μL, 3.00 mmol) was slowly added dropwise. After the dropwise addition, stirring was continued at the same temperature for 10 min. Subsequently, a solution of sodium nitrite (174 mg, 2.5 mmol) dissolved in water (500 μL) was added dropwise to the reaction system while keeping the temperature unchanged, and stirring was continued for 1 h after completion of the dropwise addition. After completion of the reaction, a solution of sodium hydroxide (377 mg, 9.4 mmol) dissolved in water (2 mL) was added slowly and the mixture was stirred for 1 h at room temperature. The reaction solution was transferred to a partition funnel and separated by extraction with ethyl acetate (10 mL) and water (10 mL), and the aqueous phase was extracted once more with ethyl acetate (10 mL). The organic phases were combined and washed sequentially with 5% aqueous sodium sulfite (5 mL) and saturated saline (10 mL), then dried with anhydrous sodium sulfate. The solvent was removed by distillation under reduced pressure to afford the target product 2,3-dibromo-5-methylpyridine (244 mg, 0.97 mmol) as a yellow solid in 97% yield. The product was analyzed by 1H-NMR (CDCl3, TMS, 300 MHz) δ (ppm): 2.30 (s, 3H), 7.73 (d, 1H, J = 1.5 Hz), 8.14 (d, 1H, J = 1.2 Hz); 13C-NMR (CDCl3, TMS, 300 MHz) δ (ppm): 17.41, 123.16. 134.13, 140.39, 142.29, 148.57; high-resolution mass spectrometry (C6H5Br2N) Theoretical: [M+] 248.8789, Measured: [M+] 248.8786; Melting point 54.2 °C.

17282-00-7
332 suppliers
$5.00/1g

29232-39-1
204 suppliers
$10.00/1g
Yield: 97%
Reaction Conditions:
Stage #1:3-bromo-5-methylpyridin-2-amine with hydrogen bromide in water
Stage #2: with bromine in water at 0 - 5; for 0.166667 h;
Stage #3: with sodium hydroxide;sodium nitritemore than 3 stages;
Steps:
32.5
In a 30-ml three-necked flask, 2-amino-3-bromo-5- methylpyridine (187 mg, 1.00 mmol) ) was charged, 48% aqueous hydrogen bromide solution (1 ml) was added and 2-amino-3- bromo-5-methylpyridine was dissolved. Then, the mixture was ice-cooled, and bromine (154 μl, 3.00 mmol) was slowly added dropwise thereto at the internal temperature of 2 to 5°C. The mixture was stirred at the same temperature for 10 minutes. To this solution, sodium nitrite (174 mg, 2.5 mmol) /water (500 μl) solution was added dropwise at the same temperature, and the mixture was stirred for 1 hour. Sodium hydroxide (377 mg, 9.4 mmol) /water (2 ml) was slowly added dropwise thereto. The mixture was stirred at room temperature for 1 hour. The solution was poured into a separating funnel. The organic layer was separated by extraction using ethyl acetate (10 ml)
References:
TAKEDA PHARMACEUTICAL COMPANY LIMITED WO2008/16184, 2008, A1 Location in patent:Page/Page column 187-188
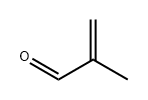
78-85-3
177 suppliers
$52.79/5ml
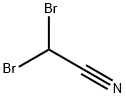
3252-43-5
116 suppliers
$19.00/25g

29232-39-1
204 suppliers
$10.00/1g