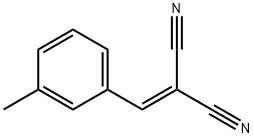
2-(3-METHYLBENZYLIDENE)-MALONONITRILE synthesis
- Product Name:2-(3-METHYLBENZYLIDENE)-MALONONITRILE
- CAS Number:15728-26-4
- Molecular formula:C11H8N2
- Molecular Weight:168.19
Yield:15728-26-4 98%
Reaction Conditions:
with amine-functionalized mesoporous silica nanospheres in water at 100; for 0.0833333 h;Microwave irradiation;Sealed tube;Knoevenagel Condensation;
Steps:
2.3 Activity Test
General procedure: The Knoevenagel condensation of aromatic aldehyde withethyl cyanoacetate was carried out under microwave irradiation.Microwave experiments were carried out with asingle mode cavity Discover Microwave synthesizer withPC control (CEM Corporation, NC, USA). The microwavemethod was generally power-controlled where reactionsmixture were irradiated with the maximum power output(300 W), achieving temperature as measured by an infraredprobe. In a typical experiment, a tube equipped with amagnetic stirrer was charged with 2.0 mmol aldehyde,2.4 mmol ethyl cyanoacetate, solid base catalyst andn-decane as an internal standard in 1.0 mL water. This tubewas sealed and the content was subjected to focusedmicrowave irradiation for up to 12-18 min at 300 W, with100 °C as the general final temperature. Then, the reactionmixture was cooled to room temperature and diluted with5.0 mL of ethyl acetate and stirred until the catalyst wasprecipitated. Organic phase was analyzed by GC (Agilent1790) equipped with a FID and a JWDB-5 95 % dimethyl-1-(5 %)-diphenylpolysiloxane column. The column temperaturewas programmed from 80 to 250 °C at a ramp of10 °C/min. N2 was used as carrier gas. The conversion was calculated based on aromatic aldehyde since the otherreactant was excess. The reproducibility was checked byrepeating each result at least three times and was found tobe within acceptable limits (±5 %).In order to determine the catalyst durability, the catalystwas allowed to settle down after each run of reactions andthe clear supernatant liquid was decanted slowly. Thecatalysts were washed thoroughly with water and ethanol,respectively, followed by vacuum drying at 80 °C overnight.Then, the catalysts were reused with fresh charge ofreactants for subsequent recycle under the identical reactionconditions.
References:
Zhu, Fengxia;Sun, Xiaojun;Lou, Fengwen;An, Litao;Zhao, Pusu [Catalysis Letters,2015,vol. 145,# 4,art. no. 1484,p. 1072 - 1079]
587-03-1
197 suppliers
$21.00/5g

109-77-3
453 suppliers
$10.00/5g

15728-26-4
19 suppliers
inquiry
620-23-5
325 suppliers
$5.00/5g
90470-72-7
1 suppliers
inquiry

109-77-3
453 suppliers
$10.00/5g

15728-26-4
19 suppliers
inquiry

620-23-5
325 suppliers
$5.00/5g

587-03-1
197 suppliers
$21.00/5g

15728-26-4
19 suppliers
inquiry