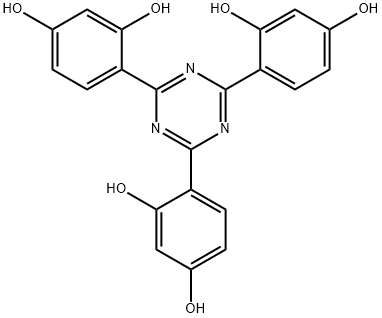
2,4,6-tris(2,4-dihydroxyphenyl)-1,3,5-triazine synthesis
- Product Name:2,4,6-tris(2,4-dihydroxyphenyl)-1,3,5-triazine
- CAS Number:2125-23-7
- Molecular formula:C21H15N3O6
- Molecular Weight:405.36
Yield:2125-23-7 89.88%
Reaction Conditions:
with hydrogenchloride;aluminum (III) chloride in water;nitrobenzene at 5 - 95;
Steps:
4 Preparation of intermediate 2,4,6-tris(2,4-dihydroxyphenyl)-1,3,5-triazine
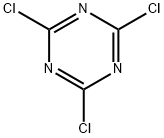
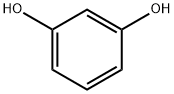
Add 35.2 grams (0.32 mol) of resorcinol, 21.4 grams (0.16 mol) of solid aluminum trichloride, and 222 grams of nitrobenzene to a 500ml four-neck flask equipped with a stirrer, thermometer, condenser, and hydrogen chloride absorption device. , Stir and raise the temperature until all the materials are dissolved, and cool down to 5-10 in water bath and ice water bath. Turn on the hydrogen chloride absorption device, add 18.5 grams (0.1 mol) of cyanuric chloride to the four-necked flask, and keep the temperature for 60 minutes. Slowly increase the temperature to the range of 10-20°C and keep it in a cold water bath for 60 minutes, then increase the temperature to 25-30°C for 60 minutes, and then take 30 minutes to increase the temperature to the set reaction temperature of 90-95°C, and keep the temperature for 3.0 hours.The reaction solution after the reaction was slowly added to a 1000 ml four-neck flask with a device stirrer and thermometer pre-added with 52 grams of 35% hydrochloric acid, and the hydrolysis temperature was controlled to be maintained at 70-80°C for 1.0 hour to terminate the reaction. Add 3.7g of ethylenediamine at this temperature, the material system gradually appears solid-liquid separation, and the temperature is as low as 40. Pour about 203 grams of liquid phase from the mouth of the four-neck bottle (GC test nitrobenzene content ≥99.0% ). Then add 330 grams of solvent DMF to the remaining materials in the 1000ml four-neck bottle, stir and warm up to all dissolve, cool the water bath to 40-45°C to precipitate solid materials, cool to 15-20°C, keep for 1.0 hour, filter, DMF wash, and dry Then, 36.4 g of intermediate 2,4,6-tris(2,4-dihydroxyphenyl)-1,3,5-triazine was obtained, with a yield of 89.88% and a purity of 98.8% by HPLC. No waste water is produced in the whole process.
References:
CN111892548,2020,A Location in patent:Paragraph 0076-0078