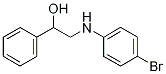
2-(4-Bromoanilino)-1-phenyl-1-ethanol synthesis
- Product Name:2-(4-Bromoanilino)-1-phenyl-1-ethanol
- CAS Number:91851-17-1
- Molecular formula:C14H14BrNO
- Molecular Weight:292.17
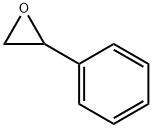
96-09-3
240 suppliers
$14.00/5g

106-40-1
493 suppliers
$12.00/5g

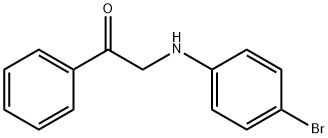
91851-17-1
8 suppliers
$60.00/100mg
Yield:91851-17-1 92%
Reaction Conditions:
with bismuth sulfide microspheres in neat (no solvent) at 60; for 2.5 h;chemoselective reaction;
Steps:
Ring opening of epoxides
General procedure: In a typical epoxide ring-opening reaction, the catalyst, Bi2S3 microspheres(30 mg), was added into a solution of 2-phenyloxirane (1mmol) and corresponding nucleophie. The solution was stirred at 60 °Cunder solvent-free conditions and the progress of the reaction wasmonitored using TLC. After completion of reaction, ethyl acetate (20mL) was added and filtrated. Finally, the crude product was subjected tocolumn chromatography on silica gel to obtain the pure product.
References:
Ghorbani-Choghamarani, Arash;Taherinia, Zahra [Molecular catalysis,2021,vol. 499,art. no. 111283]
4831-21-4
21 suppliers
$60.00/100mg

91851-17-1
8 suppliers
$60.00/100mg
![[(4-BROMOPHENYL)AMINO]ACETIC ACID](/StructureFile/ChemBookStructure3/GIF/CB3351121.gif)
13370-62-2
39 suppliers
$85.00/250mg

100-52-7
978 suppliers
$20.37/250g

91851-17-1
8 suppliers
$60.00/100mg
![Ethanone, 2-[(4-bromophenyl)imino]-1-phenyl-](/CAS/20180601/GIF/19027-81-7.gif)
19027-81-7
0 suppliers
inquiry

91851-17-1
8 suppliers
$60.00/100mg

106-40-1
493 suppliers
$12.00/5g

91851-17-1
8 suppliers
$60.00/100mg