
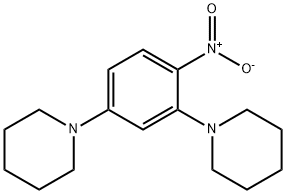
2,4-DI-PIPERIDIN-1-YL-PHENYLAMINE synthesis
- Product Name:2,4-DI-PIPERIDIN-1-YL-PHENYLAMINE
- CAS Number:436096-88-7
- Molecular formula:C16H25N3
- Molecular Weight:259.39
53013-41-5

436096-88-7
Example 23 5-(N-hydroxyformamidinyl)-furan-2-carboxylic acid (2,4-di-piperidin-1-yl-phenyl)-amide was synthesized as follows: a) Preparation of S-cyano-furan-1-carboxylic acid (2,4-di-piperidin-1-yl-phenyl)-amide: Piperidine (3.35 g, 39.4 mmol) was added dropwise to a solution of 2,4-difluoronitrobenzene (2.09 g, 13.1 mmol) in ethanol (10 mL) at room temperature. The reaction mixture was stirred overnight and then concentrated under vacuum. The residue was dissolved in ethyl acetate (100 mL), washed with water (2 x 100 mL), dried over anhydrous sodium sulfate and concentrated again under vacuum. Purification by silica gel column chromatography (10% ethyl acetate-hexane) gave 610 mg (16% yield) of 2,4-dipiperidinyl nitrobenzene as an oil. Subsequently, 2,4-dipiperidinylnitrobenzene (273 mg, 0.94 mmol) was stirred in methanol (10 mL) in the presence of 5% Pd-C (168 mg) under hydrogen atmosphere for 2 hours. The reaction mixture was filtered through diatomaceous earth and concentrated in vacuum to give 230 mg (94% yield) of 2,4-dipiperidinylaniline as an oil. Using a method similar to step (c) of Example 4, 2,4-dipiperidinylaminobenzene (100 mg, 0.38 mmol) was reacted with 5-cyanofuran-2-carbonyl chloride (73 mg, 0.46 mmol) in the presence of N,N-diisopropylethylamine (DIEA, 145 μL, 0.83 mmol) to give 90 mg (62% yield) of 2,4 -bis(piperidin-1-yl)aniline as a yellow solid. Mass spectrum (ESI, m/z): calculated value of C22H26N4O2 was 379.2 (M + H) and measured value was 379.2.

53013-41-5
4 suppliers
$28.60/250mg

436096-88-7
25 suppliers
$60.00/100mg
Yield:436096-88-7 94%
Reaction Conditions:
with hydrogen;5%-palladium/activated carbon in methanol; for 2 h;
Steps:
23.a
Example 23 5-(N-Hydroxycarbamimidoyl)-furan-2-carboxylic acid (2,4-di-piperidin-l-yl-phenyl)- amide a) S-Cyano-furan-l-carboxylic acid (2,4-di-piperidin-l-yl-phenyl)-arnide; To a solution of 2,4-difluoronitrobenzene (2.09 g, 13.1 mmol) in EtOH (10 mL) at ambient temperature was added piperidine (3.35 g, 39.4 mmol) dropwise. The reaction was allowed to stir overnight and concentrated in vacuo. The residue was dissolved in EtOAc (100 mL), washed with water (2 x 100 mL), dried (Na2SO4) and concentrated in vacuo. Purification by silica gel column chromatography (10% EtOAc-hexane) afforded 610 mg EPO
References:
WO2006/47504,2006,A1 Location in patent:Page/Page column 68-69