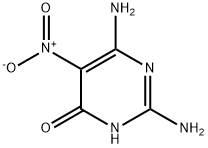
2,4-Diamino-6-hydroxy-5-nitropyrimidine synthesis
- Product Name:2,4-Diamino-6-hydroxy-5-nitropyrimidine
- CAS Number:3346-23-4
- Molecular formula:C4H5N5O3
- Molecular Weight:171.11
56-06-4

3346-23-4
General procedure for the synthesis of 2,6-diamino-5-nitropyrimidin-4-ol from 2,6-diaminopyrimidin-4(1H)-one: 126 g of 2,6-diaminopyrimidin-4(1H)-one was dissolved in 730 g of the filtrate from the above batch with stirring, and the temperature of the reaction was controlled to be between 30 and 35°C. Subsequently, 20 g of concentrated sulphuric acid with a mass concentration of 98% was slowly added. Under the condition of maintaining the same temperature, 68.5 g (1.01 mol) of 93% fuming nitric acid was added dropwise. After the dropwise addition, the reaction was continued for 2 hours. Upon completion of the reaction, the reaction mixture was cooled to -5 to 0°C to promote crystallization, followed by filtration to obtain about 730 g of brown-red filtrate, which can be used as a solvent for the next batch of the reaction. The solid obtained by filtration was first washed with 100 mL of dichloromethane and then with 200 mL of potable water, and drained to obtain 225 g (165 g on a dry weight basis) of brownish-yellow to brownish-red solid, i.e., the target product 2,6-diamino-5-nitropyrimidin-4-ol (molecular weight 171.11). The purity of the product was 98.7% by HPLC analysis, and the yield was 96.4% as 2,6-diaminopyrimidin-4(1H)-one.

56-06-4
504 suppliers
$5.00/25mg

3346-23-4
33 suppliers
inquiry
Yield: 96.4%
Reaction Conditions:
with sulfuric acid;nitric acid at 30 - 35; for 2 h;
Steps:
2 Example 2 Preparation of 2,4-Diamino-5-nitro-6-hydroxypyrimidine
Dissolve 126 g of diaminopyrimidine730 g of the above batch of filtrate, stirring, temperature control 30 ~ 35 °C,Add 20g of concentrated sulfuric acid,The mass concentration is 98%.After the dropwise addition, 68.5 g (1.01 mol) of 93% fuming nitric acid was continuously added dropwise at 30 to 35°C.After dripping, continue the incubation for 2 hours.After the reaction is completed, cool and crystallize to -5~0°C and filter.About 730 g of brownish red filtrate was obtained as the next batch of reaction solvent.The cake was washed with 100 mL of dichloromethane,200mL drinking water washing, draining,225 g (of 165 g) was obtained as a tan-yellow-brown red solid, i.e. a nitropyrimidine (MW 171.11) wet product.HPLC purity 98.7%, measured as diaminopyrimidine,The yield was 96.4%.
References:
Weifang Aotong Pharmaceutical Co., Ltd.;Yang Zaigen;Li Xi;Yan Jinhua;Zhou Zhiwei CN107903215, 2018, A Location in patent:Paragraph 0039-0050