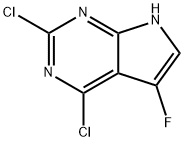
2,4-DICHLORO-5-FLUORO-7H-PYRROLO[2,3-D]PYRIMIDINE synthesis
- Product Name:2,4-DICHLORO-5-FLUORO-7H-PYRROLO[2,3-D]PYRIMIDINE
- CAS Number:1053228-29-7
- Molecular formula:C6H2Cl2FN3
- Molecular Weight:206
90213-66-4
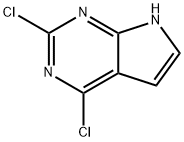
![2,4-DICHLORO-5-FLUORO-7H-PYRROLO[2,3-D]PYRIMIDINE](/CAS/GIF/1053228-29-7.gif)
1053228-29-7
2,4-Dichloro-7H-pyrrolo[2,3-d]pyrimidine (200 mg, 1.06 mmol) was used as starting material and dissolved in acetonitrile (5.0 mL). Subsequently, 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2]octanebis(tetrafluoroborate) (561.6 mg, 1.6 mmol) and acetic acid (1 mL) were added sequentially to this solution. The reaction mixture was heated at 80 °C and stirred continuously for 24 hours. Upon completion of the reaction, the organic layer was separated, dried with anhydrous magnesium sulfate, filtered to remove the desiccant, and the filtrate was subsequently concentrated under reduced pressure. The residue was purified by column chromatography to afford the target compound 2,4-dichloro-5-fluoro-7H-pyrrolo[2,3-d]pyrimidine (170.0 mg, 80.1% yield). The product was confirmed by NMR hydrogen spectroscopy (500 MHz, CD3OD) with a chemical shift δ of 7.36 (s, 1H).

90213-66-4
494 suppliers
$8.00/250mg
![2,4-DICHLORO-5-FLUORO-7H-PYRROLO[2,3-D]PYRIMIDINE](/CAS/GIF/1053228-29-7.gif)
1053228-29-7
66 suppliers
$174.00/100mg
Yield: 80.1%
Reaction Conditions:
with acetic acid;Selectfluor in acetonitrile at 80; for 24 h;
Steps:
82.1 Step 1: Preparation of 2,4-dicMoro-5-fluoro-7H^yrrolo[2,3-d]pyrimidine
After 2,4-dicMoro-7H-pyrrolo[2,3-d]pyrimidine (200 mg, 1.06 mmol) was dissolved in acetonitrile (5.0 mL), l-chloromethyl-4-fluoro-l,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) (561.6 mg, 1.6 mmol) and acetic acid (1 mL) were added thereto. The mixture was heated at 80 °C and stirred for 24 hours, and then the organic layer was isolated, treated with magnesium sulfate, filtered and then concentrated under reduced pressure. The residue was isolated by column chromatography to obtain a title compound (170.0 mg, yield: 80.1%). (0795) NMR (500MHz,CD3OD) δ 7.36(s, 1H)
References:
DAEWOONG PHARMACEUTICAL CO., LTD.;KIM, In Woo;HAN, Mi Ryeong;YOO, Jakyung;OH, Yun Ju;KIM, Ji Duck;KIM, Nam Youn;JUN, Sun Ah;LEE, Jun Hee;PARK, Joon Seok WO2018/4306, 2018, A1 Location in patent:Page/Page column 91

90213-66-4
494 suppliers
$8.00/250mg
![2,4-DICHLORO-5-FLUORO-7H-PYRROLO[2,3-D]PYRIMIDINE](/CAS/GIF/1053228-29-7.gif)
1053228-29-7
66 suppliers
$174.00/100mg
![2,4,5-Trichloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS2/GIF/1053228-28-6.gif)
1053228-28-6
91 suppliers
$28.00/100mg
![7H-Pyrrolo[2,3-d]pyrimidine-2,4-diol](/CAS/GIF/39929-79-8.gif)
39929-79-8
197 suppliers
$8.00/250mg
![2,4-DICHLORO-5-FLUORO-7H-PYRROLO[2,3-D]PYRIMIDINE](/CAS/GIF/1053228-29-7.gif)
1053228-29-7
66 suppliers
$174.00/100mg