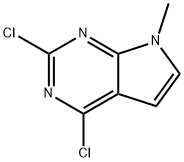
2,4-dichloro-7-Methyl-7H-pyrrolo[2,3-d]pyriMidine synthesis
- Product Name:2,4-dichloro-7-Methyl-7H-pyrrolo[2,3-d]pyriMidine
- CAS Number:90213-67-5
- Molecular formula:C7H5Cl2N3
- Molecular Weight:202.04
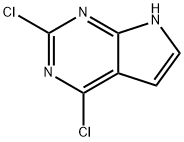
90213-66-4

74-88-4
![2,4-dichloro-7-Methyl-7H-pyrrolo[2,3-d]pyriMidine](/CAS/GIF/90213-67-5.gif)
90213-67-5
To a suspension of sodium hydride (480 mg, 12.00 mmol, 1.10 eq., 60% dispersed in mineral oil) in tetrahydrofuran (50 mL) was slowly added dropwise a solution of 2,4-dichloro-7H-pyrrolo[2,3-d]pyrimidine (2 g, 10.64 mmol, 1.00 eq.) in tetrahydrofuran (50 mL). The reaction mixture was stirred at 0 °C for 30 min and then iodomethane (1.66 g, 11.70 mmol, 1.10 eq.) was added dropwise at the same temperature. After addition, the reaction system was brought to room temperature and stirred overnight. Upon completion of the reaction, the reaction was quenched by the addition of water (20 mL) to the mixture and subsequently extracted with ethyl acetate. The combined organic phases were washed with saturated saline, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography with ethyl acetate/petroleum ether (1:5, v/v) as eluent to afford 2.1 g (98% yield) of the title compound, 2,4-dichloro-7-methyl-7H-pyrrolo[2,3-d]pyrimidine, as a white solid.LC-MS (ESI, m/z): 202 [M+H]+.

90213-66-4
496 suppliers
$8.00/250mg

74-88-4
356 suppliers
$15.00/10g
![2,4-dichloro-7-Methyl-7H-pyrrolo[2,3-d]pyriMidine](/CAS/GIF/90213-67-5.gif)
90213-67-5
68 suppliers
$10.00/100mg
Yield: 98%
Reaction Conditions:
Stage #1:2,4-dichloro-7H-pyrrolo[2,3-d]pyrimidine with sodium hydride in tetrahydrofuran;acetonitrile at 0; for 0.5 h;
Stage #2:methyl iodide in tetrahydrofuran;acetonitrile at 0 - 20;
Steps:
HH.1; VV.1 Step 1: Synthesis of 2,4-dichloro-7-methyl-7H-pyrrolo[2,3-d]pyrimidine
To a solution of sodium hydride (480 mg, 12.00 mmol, 1.10 equiv, 60%) in tetrahydrofuran (50 mL), a solution of 2,4-dichloro-7H-pyrrolo[2,3-d]pyrimidine (2 g, 10.64 mmol, 1.00 equiv) in tetrahydrofuran (50 mL) was slowly added.
The resulting solution was stirred at 0° C. for 30 min followed by the addition of methyl iodide (1.66 g, 11.70 mmol, 1.10 equiv) at 0° C.
The mixture was stirred at room temperature overnight.
After completion, 20 mL of water was added to the mixture and the solution was extracted with ethyl acetate and washed with brine.
The organic phase was dried over anhydrous sodium sulfate, filtered and then concentrated under vacuum.
The residue was purified by silica gel column chromatography eluting with ethyl acetate/petroleum ether (1:5) to afford 2.1 g (98%) of 2,4-dichloro-7-methyl-7H-pyrrolo[2,3-d]pyrimidine as a white solid. LC-MS (ES, m/z): 202 [M+H]
References:
Genentech, Inc.;Blaquiere, Nicole;Castanedo, Georgette;Feng, Jianwen A.;Hu, Baihua;Staben, Steven;Yuen, Po-wai;Wu, Guosheng;Lin, Xingyu;Burch, Jason US2015/57260, 2015, A1 Location in patent:Paragraph 0436; 0437; 0588; 0589

90213-66-4
496 suppliers
$8.00/250mg
![2,4-dichloro-7-Methyl-7H-pyrrolo[2,3-d]pyriMidine](/CAS/GIF/90213-67-5.gif)
90213-67-5
68 suppliers
$10.00/100mg
![2-Amino-4-chloropyrrolo[2,3-d]pyrimidine](/CAS/GIF/84955-31-7.gif)
84955-31-7
206 suppliers
$5.00/250mg
![2,4-dichloro-7-Methyl-7H-pyrrolo[2,3-d]pyriMidine](/CAS/GIF/90213-67-5.gif)
90213-67-5
68 suppliers
$10.00/100mg
52133-67-2
195 suppliers
$8.00/1g
![2,4-dichloro-7-Methyl-7H-pyrrolo[2,3-d]pyriMidine](/CAS/GIF/90213-67-5.gif)
90213-67-5
68 suppliers
$10.00/100mg
![7H-Pyrrolo[2,3-d]pyrimidine-2,4-diol](/CAS/GIF/39929-79-8.gif)
39929-79-8
199 suppliers
$8.00/250mg
![2,4-dichloro-7-Methyl-7H-pyrrolo[2,3-d]pyriMidine](/CAS/GIF/90213-67-5.gif)
90213-67-5
68 suppliers
$10.00/100mg