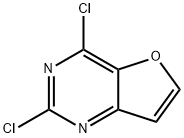
2,4-DICHLOROFURO[3,2-D]PYRIMIDINE synthesis
- Product Name:2,4-DICHLOROFURO[3,2-D]PYRIMIDINE
- CAS Number:956034-07-4
- Molecular formula:C6H2Cl2N2O
- Molecular Weight:189
![FURO[3,2-D]PYRIMIDINE-2,4-DIOL](/CAS/GIF/956034-06-3.gif)
956034-06-3
![2,4-DICHLOROFURO[3,2-D]PYRIMIDINE](/CAS/GIF/956034-07-4.gif)
956034-07-4
Furo[3,2-d]pyrimidine-2,4-diol (7J) (1.52 g, 10 mmol) was added to N,N-dimethylaniline (1 mL, 8 mmol) and trichlorophosphorus (0.9 mL, 9.65 mmol) as a starting material and the reaction was carried out for 3 hours at 130°C. The reaction was carried out at 130°C for 3 hours. Upon completion of the reaction, the mixture was cooled to room temperature and carefully quenched with ice water. The resulting solid product was collected by filtration, washed with water and dried under vacuum to afford 2,4-dichlorofuro[3,2-d]pyrimidine (7K) (1.02 g, 54% yield) as a light brown solid. The melting point of the product was 107.3°C. The NMR hydrogen spectrum (300 MHz, CDCl3) data were as follows: δ 8.08 (d, J = 2.2 Hz, 1H), 7.02 (d, J = 2.2 Hz, 1H).
![FURO[3,2-D]PYRIMIDINE-2,4-DIOL](/CAS/GIF/956034-06-3.gif)
956034-06-3
35 suppliers
inquiry
![2,4-DICHLOROFURO[3,2-D]PYRIMIDINE](/CAS/GIF/956034-07-4.gif)
956034-07-4
140 suppliers
$50.00/100mg
Yield:956034-07-4 87%
Reaction Conditions:
with N,N-dimethyl-aniline;trichlorophosphate at 130; for 2 h;
Steps:
10; vii
To a mixture of lH-furo[3,2-d]pyrimidine-2,4-dione (3.0 g, 0.020 mol) in NJf- dimethylaniline (2.0 mL, 0.016 mol) was added phosphorous oxychloride (18.0 mL, 0.193 mol) and the resulting mixture heated at 130 °C for 2 h. The resulting black solution was cooled to RT, then carefully quenched with crushed ice and H2O, then extracted with EtOAc.The organic layer was isolated, dried (MgSO4) and concentrated in vacuo to give the title compound as a tan solid (3.26 g, 87 %). 1H NMR (400 MHz, CDCl3): δ 7.02 (d, J = 2.2 Hz, 1 H) and 8.08 (d, J = 2.2 Hz, 1 H).
References:
F.HOFFMANN-LA ROCHE AG WO2008/152394, 2008, A1 Location in patent:Page/Page column 25; 35
![FURO[3,2-D]PYRIMIDINE-2,4-DIOL](/CAS/GIF/956034-06-3.gif)
956034-06-3
35 suppliers
inquiry
![2,4-DICHLOROFURO[3,2-D]PYRIMIDINE](/CAS/GIF/956034-07-4.gif)
956034-07-4
140 suppliers
$50.00/100mg
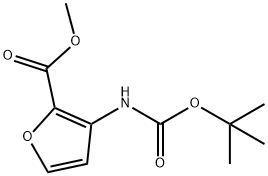
956034-03-0
39 suppliers
$84.00/100mg
![2,4-DICHLOROFURO[3,2-D]PYRIMIDINE](/CAS/GIF/956034-07-4.gif)
956034-07-4
140 suppliers
$50.00/100mg
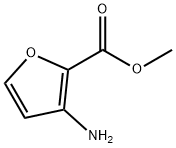
956034-04-1
136 suppliers
$49.00/100mg
![2,4-DICHLOROFURO[3,2-D]PYRIMIDINE](/CAS/GIF/956034-07-4.gif)
956034-07-4
140 suppliers
$50.00/100mg
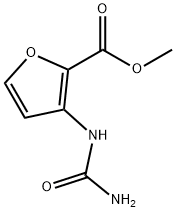
1093066-63-7
10 suppliers
inquiry
![2,4-DICHLOROFURO[3,2-D]PYRIMIDINE](/CAS/GIF/956034-07-4.gif)
956034-07-4
140 suppliers
$50.00/100mg