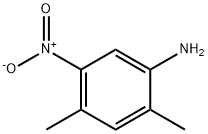
2,4-DIMETHYL-5-NITROANILINE synthesis
- Product Name:2,4-DIMETHYL-5-NITROANILINE
- CAS Number:2124-47-2
- Molecular formula:C8H10N2O2
- Molecular Weight:166.18
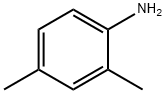
95-68-1

2124-47-2
The general procedure for the synthesis of 2,4-dimethyl-5-nitroaniline from 2,4-dimethylaniline is as follows: nitric acid (33 g) was added slowly and dropwise to a stirring solution of 2,4-dimethylaniline (40 g, 0.33 mmol) in concentrated hydrochloric acid over a period of 3 h, keeping the reaction temperature below 15 °C. The reaction temperature was kept below 15 °C for the first time. Subsequently, sulfuric acid (400 g) was added. After addition, the reaction mixture was continued to be stirred at 15 °C for 1 h. The reaction was then poured into ice (600 mL), stirred for 30 min and filtered. The resulting yellow filter cake was neutralized with saturated aqueous sodium bicarbonate (500 mL) and subsequently extracted with ethyl acetate (3 x 200 mL). The organic extracts were combined, dried with magnesium sulfate, filtered and the solvent was removed in vacuum to give the crude product. The crude product was recrystallized by ethanol-water mixed solvent to give a final orange solid product (39 g, 71% yield, containing 20% dinitro by-product). The product was analyzed by IR showing characteristic absorption peaks (Nujol, cm-1): 3469, 3386, 3239, 2956, 2925, 2855, 1719, 1636, 1514, 1461, 1377, 1339, 1297, 1273, 1222, 1170, 1034, 992, 885, 870, 849. 805, 758, 745, 723, 640, 607 and 571; 1H NMR (400 MHz, CDCl3) δ: 7.15 (1H, s), 6.87 (1H, s), 4.99 (2H, br.s), 2.21 (3H, s), 1.97 (3H, s).
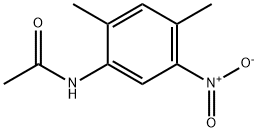
62476-60-2
3 suppliers
inquiry

2124-47-2
136 suppliers
$12.00/250mg
Yield:-
Reaction Conditions:
with sulfuric acid in ethanol;water
Steps:
2.H.3 3.
3. Preparation of 2,4-dimethyl-5-nitroaniline A mixture of N-(2,4-dimethyl-5-nitrophenyl)acetamide, water (24 mL), concentrated sulfuric acid (12 mL), and ethanol (120 mL) was heated at reflux under an atmosphere of nitrogen for 4 hours. The ethanol was evaporated under reduced pressure and the residue partitioned between ethyl acetate (250 mL) and brine (150 mL). The brine solution was reextracted with ethyl acetate. The combined ethyl acetate extracts were then dried over magnesium sulfate. Evaporation afforded a crude product which was flash chromatographed on silica and eluted with ethyl acetate:hexane (1:4) to afford 4.63 g as a pale yellow solid.
References:
F. HOFFMANN-LA ROCHE AG EP887346, 1998, A2

95-68-1
314 suppliers
$14.00/5g

2124-47-2
136 suppliers
$12.00/250mg
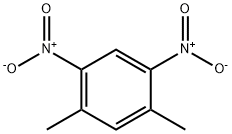
616-72-8
35 suppliers
$45.00/25mg

2124-47-2
136 suppliers
$12.00/250mg