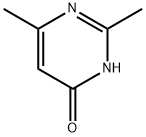
2,4-DIMETHYL-6-HYDROXYPYRIMIDINE synthesis
- Product Name:2,4-DIMETHYL-6-HYDROXYPYRIMIDINE
- CAS Number:6622-92-0
- Molecular formula:C6H8N2O
- Molecular Weight:124.14
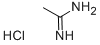
124-42-5

141-97-9

6622-92-0
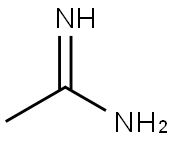
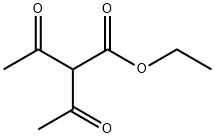
General procedure for the synthesis of 2,4-dimethyl-6-hydroxypyrimidines from acetamidine hydrochloride and ethyl acetoacetate: to a solution of sodium ethanolate (10.5 g, 154 mmol) in ethanol (200 mL) was added ethyl 3-oxobutanoate (10.0 g, 77 mmol) and acetamidine hydrochloride (7.23 g, 77 mmol) sequentially. The reaction mixture was heated to reflux for 20 hours. After completion of the reaction, water (10 mL) was added to the mixture. The reaction mixture was concentrated under reduced pressure to give the crude product. The crude product was purified by column chromatography (silica gel as stationary phase, dichloromethane/methanol=15:1 as mobile phase) to afford 2,6-dimethylpyrimidin-4-ol as a yellow solid (3.43 g, 36% yield).LRMS (M+H+) m/z: Calculated value 124.06; measured value 124.
Yield:6622-92-0 36%
Reaction Conditions:
with sodium ethanolate in ethanol; for 20 h;Reflux;
Steps:
53.1 2,6-dimethylpyrimidin-4-ol.
2,6-dimethylpyrimidin-4-ol. Ethyl 3-oxobutanoate (10.0 g, 77 mmol) and acetimidamide hydrochloride (7.23 g, 77 mmol) were added to the solution of sodium ethanolate (10.5 g, 154 mmol) in ethanol (200 mL). And the mixture was refluxed for 20 hours. Water (10 mL) was added to the mixture. The resultant mixture was concentrated to give a residue. The residue was purified by column chromatography (silica gel, dichloromethane /methanol = 15: 1) to give 2,6-dimethylpyrimidin-4-ol as a yellow solid (3.43 g, 36%). LRMS (M + H+) m/z: calcd 124.06; found 124.
References:
WO2013/75083,2013,A1 Location in patent:Paragraph 00374; 00375