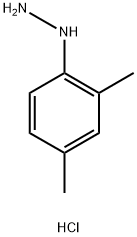
2,4-Dimethylphenylhydrazine hydrochloride synthesis
- Product Name:2,4-Dimethylphenylhydrazine hydrochloride
- CAS Number:60480-83-3
- Molecular formula:C8H13ClN2
- Molecular Weight:172.66
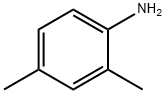
95-68-1

60480-83-3
Example 13, Preparation of 2,4-dimethylphenylhydrazine hydrochloride (Compound 13): to a mixed solution of 2,4-dimethylaniline (35.4 mL; 36.3 g; 0.3 mol) cooled to 0 °C and concentrated hydrochloric acid (220 mL), aqueous sodium nitrite (20.6 g; 0.3 mol dissolved in 60 mL of water) was slowly added dropwise with a controlled rate of acceleration of dropwise addition to maintain the temperature of the reaction at a temperature not The rate of acceleration was controlled to maintain the reaction temperature not exceeding 5 °C. Upon completion of the reaction, the resulting solution was filtered and added to a solution of stannous chloride (124 g; 0.65 mol) in concentrated hydrochloric acid (124 mL) that had been pre-cooled to 5 °C with vigorous stirring. Subsequently, the resulting precipitate was separated by filtration, suspended in water (500 mL) and adjusted to pH 12 with sodium hydroxide solution (40%).The reaction mixture was extracted with ether (3 x 200 mL), the ether layers were combined and dried over anhydrous magnesium sulfate. The dried ether solution was passed through dry HCl gas until the precipitation was complete. The precipitate was collected by filtration, washed with ether and dried in a vacuum desiccator using phosphorus pentoxide as desiccant to give 2,4-dimethylphenylhydrazine hydrochloride (compound 13) in 18.5 g (35.8% yield). UV maximum absorption wavelength λmax 281 nm. mass spectrum (MALDI) (C8H12N2): measured value m/z 137.2, calculated value m/z 136.19. 1H-NMR (D2O), δ (ppm): 2.03 (3H, s, para-CH3), 2.08 (3H, s, o- CH3), 6.72 (1H, s, aryl ring H ), 6.93 (2H, d, aryl ring H).

95-68-1
314 suppliers
$14.00/5g

60480-83-3
171 suppliers
$10.00/500mg
Yield:60480-83-3 35.8%
Reaction Conditions:
Stage #1:2,4-Xylidine with hydrogenchloride;sodium nitrite in water at 0 - 5;
Stage #2: with tin(ll) chloride in water at 5;
Steps:
13
Example 13, Preparation of 2.4-xylylhydrazine hydrochloride (compound 13) To a mixture of 2.4-dimethylaniline (35.4 mL; 36.3 g; 0.3 mole) and concentrated hydrochloric acid (220 mL) chilled to 0°C, the solution of sodium nitrite (20.6 g; 0.3 mole) in water (60 mL) was added dropwise at such a rate to maintain reaction temperature not higher than 5°C. Then, the solution prepared was filtered off and added to a solution of stannous chloride (124 g; 0.65 mole) in concentrated hydrochloric acid (124 mL) chilled to 5°C, while vigorous stirring. The precipitate formed was separated by filtration, slurried in water (500 mL), and alkalized to pH 12 with solution of sodium hydroxide (440%). The reaction mixture was extracted with diethyl ether (3*200 mL). Ethereal extracts were dried over MgSO4. Then, a stream of dried hydrogen chloride was bubbled via the ethereal solution of p-tolyl hydrazine. The precipitate formed was filtered, washed with ether, and dried in vacuum desiccator over P2O5. 2.4-Xylylhydrazine hydrochloride (compound 13) was obtained with yield of 18.5 g (35.8%). λmax 281 nm. Mass-spectrum (MALDI) (C8H12N2): found m/z 137.2, calculated m/z 136.19. 1H- NMR (D2O), δ (ppm): 2.03 (3H, s, p-CH3), 2.08 (3H, s, -CH3), 6.72 (1H, s, ArH), 6.93 (2H, d, ArH)
References:
UCHREZHDENIE ROSSIISKOI AKADEMII NAUK INSTITUT MOLEKULYARNOI BIOLOGII IM. V.A. ENGELGARDTA RAN (IMB RAN) EP2157088, 2010, A1 Location in patent:Page/Page column 43-44