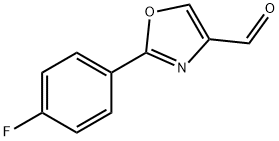
2-(4-FLUORO-PHENYL)-OXAZOLE-4-CARBALDEHYDE synthesis
- Product Name:2-(4-FLUORO-PHENYL)-OXAZOLE-4-CARBALDEHYDE
- CAS Number:152940-51-7
- Molecular formula:C10H6FNO2
- Molecular Weight:191.16
![[2-(4-FLUORO-PHENYL)-OXAZOL-4-YL]-METHANOL](/CAS/GIF/885273-80-3.gif)
885273-80-3
21 suppliers
inquiry

152940-51-7
22 suppliers
inquiry
Yield:152940-51-7 63%
Reaction Conditions:
with Dess-Martin periodane in dichloromethane at 0 - 20; for 3 h;
Steps:
9
2-(4-Fluorophenyl)oxazole-4-carbaldehydeDess-Martin periodinane (12.8 g, 30.2 mmol) was added to a solution of (2-(4- fluorophenyl)oxazol-4-yl)methanol (4.5 g, 23.29 mmol) in dry CH2CI2 (90 mL) at 0 °C. The reaction mixture was stirred at room temperature for 3 h. The reaction mixture was then quenched with saturated NaHC03 solution at 0 °C. The organic product was extracted with EtOAc. The combined extracts were washed with saturated sodium thiosulfate solution and brine. Solvent was removed under reduced pressure and the crude product was purified by column chromatography (silica gel 60-120 mesh, eluent 10% EtOAc in petroleum ether) to afford 2-(4-fluorophenyl)oxazole-4-carbaldehyde (2.8g, yield 63%) as a white solid. 1 H NMR (300 MHz, CDCI3) δ 10.01 (s, 1 H), 8.32 (s, 1 H), 8.14 - 8.10 (m, 2H), 7.23 - 7.17 (t, J = 8.8 Hz, 2H). MS (ESI) m/z: Calculated for Ci0H6FNO2: 191 .04; found: 191.8 (M+H)+
References:
WO2011/88187,2011,A1 Location in patent:Page/Page column 52

1314419-49-2
0 suppliers
inquiry

152940-51-7
22 suppliers
inquiry

56108-05-5
4 suppliers
inquiry

152940-51-7
22 suppliers
inquiry

1065102-64-8
8 suppliers
inquiry

152940-51-7
22 suppliers
inquiry