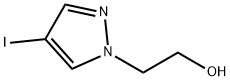
2-(4-Iodo-1H-pyrazol-1-yl)ethanol synthesis
- Product Name:2-(4-Iodo-1H-pyrazol-1-yl)ethanol
- CAS Number:1408334-75-7
- Molecular formula:C5H7IN2O
- Molecular Weight:238.03
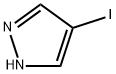
3469-69-0

540-51-2

1408334-75-7
General procedure: preparation of 2-(4-iodo-1H-pyrazol-1-yl)ethanol 4-Iodo-1H-pyrazole (4.50 g, 23.20 mmol) was dissolved in N,N-dimethylformamide (DMF, 45 mL), and sodium hydride (60% w/w, 1.42 g, 35.5 mmol) was added at 0 °C and stirred at room temperature for 1 hour. Subsequently, 2-bromoethanol (2.5 mL, 35.2 mmol) was added dropwise to the reaction mixture at 0 °C. The reaction system was warmed up to 65 °C and stirred continuously for 3 days. Upon completion of the reaction, the reaction was quenched with saturated sodium chloride solution and ethyl acetate (EtOAc) and the aqueous phase was extracted with ethyl acetate. The organic phases were combined, washed sequentially with water and saturated sodium chloride solution, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography using cyclohexane/ethyl acetate (0 to 50% gradient) as eluent to afford the target compound 2-(4-iodo-1H-pyrazol-1-yl)ethanol (3.55 g, 64% yield). 1H NMR (500 MHz, CDCl3): δ 7.55 (s, 1H), 7.52 (s, 1H), 4.32-4.22 (m, 2H), 4.04-3.95 (m, 2H), 2.79-2.68 (br m, 1H). LCMS (ESI) Rt = 1.50 min, MS m/z 238 [M + H]+.

3469-69-0
402 suppliers
$5.00/1g

540-51-2
255 suppliers
$17.00/5g

1408334-75-7
44 suppliers
$21.00/100mg
Yield:1408334-75-7 64%
Reaction Conditions:
Stage #1: 4-iodopyrazolewith sodium hydride in N,N-dimethyl-formamide at 0 - 20; for 1 h;
Stage #2: 2-bromoethanol in N,N-dimethyl-formamide at 0 - 65; for 72 h;
Steps:
57 Preparation 57: 2-(4-iodo-1 H-pyrazol-1 -yl)ethanol
Preparation 57: 2-(4-iodo-1 H-pyrazol-1 -yl)ethanol A solution of 4-iodo-1 H-pyrazole (4.50 g, 23.20 mmol) in DMF (45 mL) was treated with sodium hydride (60% w/w, 1 .42 g, 35.5 mmol) at 0°C and stirred at room temperature. After 1 hour the resulting mixture was treated with 2-bromoethanol (2.5 mL, 35.2 mmol) at 0°C. The resulting mixture was heated to 65 °C for 3 days. The reaction quenched with brine/EtOAc and the aqueous layer extracted with EtOAc. The combined organic layers were washed with water, brine, dried and concentrated. The residue was purified by silica gel column chromatography eluting with 0 to 50% EtOAc in cyclohexane to give the title compound (3.55 g, 64%). 1 H NMR (500 MHz, CDCI3): δ 7.55 (s, 1 H), 7.52 (s, 1 H), 4.32 - 4.22 (m, 2H), 4.04 - 3.95 (m, 2H), 2.79 - 2.68 (br m, 1 H). LCMS (ESI) Rt = 1 .50 minutes MS m/z 238 [M+H]+
References:
WO2014/37750,2014,A1 Location in patent:Paragraph 00142-00144
96-49-1
529 suppliers
$6.00/100g

3469-69-0
402 suppliers
$5.00/1g

1408334-75-7
44 suppliers
$21.00/100mg
![4-Iodo-1-[2-(tetrahydro-2H-pyran-2-yloxy)ethyl]-1H-pyrazole](/CAS/GIF/879487-88-4.gif)
879487-88-4
15 suppliers
inquiry

1408334-75-7
44 suppliers
$21.00/100mg