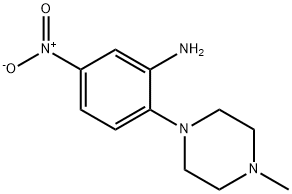
2-(4-METHYLPIPERAZIN-1-YL)-5-NITROANILINE synthesis
- Product Name:2-(4-METHYLPIPERAZIN-1-YL)-5-NITROANILINE
- CAS Number:5367-66-8
- Molecular formula:C11H16N4O2
- Molecular Weight:236.27
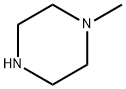
109-01-3
672 suppliers
$5.00/5G
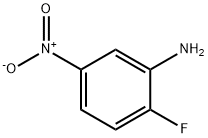
369-36-8
258 suppliers
$10.00/10g

5367-66-8
30 suppliers
$45.00/250mg
Yield:5367-66-8 82%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 80; for 16 h;
Steps:
1
To a solution of 2-fluoro-5-nitroaniline (2.0g, 12mmol) in DMF (16.0mL), Cs2C03 (8.3g, 25mmol) and N-methyl piperazine (1.53g, 15mmol) were added, and the resulting mixture was heated to 80°C for 16h. The reaction mixture was cooled to room temperature, diluted with water (50.0mL) and extracted with ethyl acetate (50.0mL). The organic layer was dried over anhydrous sodium sulfate and concentrated under reduce pressure. The residue was purified by silica gel column chromatography to obtain 1 (2.3g, 82%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6): δ 2.22 (s, 3H), 2.49 (m, 4H), 2.9 (brs, 4H), 5.2 (s, 2H), 6.98 (d, J = 8.6 Hz, 1H), 7.41-7.44 (dd, J = 2.7, 8.6 Hz, 1H), 7.52 (d, J = 2.7 Hz, 1H). MS m/z (M+H): 237.1
References:
WO2014/149164,2014,A1 Location in patent:Paragraph 00868