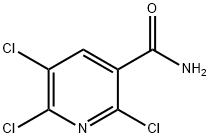
2,5,6-trichloronicotinaMide synthesis
- Product Name:2,5,6-trichloronicotinaMide
- CAS Number:142266-62-4
- Molecular formula:C6H3Cl3N2O
- Molecular Weight:225.46
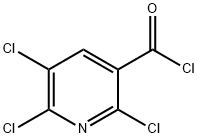
58584-88-6

142266-62-4
General procedure for the synthesis of 2,5,6-trichloropyridin-3-amide from 2,5,6-trichloronicotinoyl chloride: a solution of dioxane (20 ml) of 2,5,6-trichloronicotinoyl chloride (2.5 g, 10.2 mmol) was slowly added dropwise to 10 ml of ammonium hydroxide (28% NH3 aqueous solution) at 0 °C. After the dropwise addition, the reaction mixture was continued to be stirred at 0°C for 10 min. Subsequently, extraction was carried out with dichloromethane (3 x 50 ml). All organic phases were combined, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to afford 2,5,6-trichloropyridin-3-amide (2.3 g, 100% yield), which could be used in subsequent reactions without further purification.
54718-39-7
157 suppliers
$11.00/250mg

142266-62-4
82 suppliers
$121.00/100mg
Yield:142266-62-4 92%
Reaction Conditions:
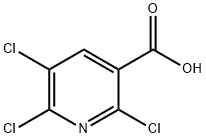
Stage #1:2,5,6-Trichloronicotinic Acid with 1,1'-carbonyldiimidazole in tetrahydrofuran at 50; for 1.08333 h;Inert atmosphere;
Stage #2: with ammonium hydroxide in toluene at 0 - 20; for 0.166667 h;
Steps:
2,5,6-Trichloronicotinamide
1,1'-Carbonyldiimidazole (40 g, 247 mmol) was added in portions to 2,5,6-trichloronicotinic acid (50.7 g, 224 mmol, Combi-Blocks, San Diego, Calif., USA) in THF (400 mL), allowing gas evolution to cease between additions.
The resulting mixture was stirred for 5 min and then was degassed with house vacuum and flushed with nitrogen (*2).
The resulting mixture was heated to 50° C. for 60 min, then diluted with toluene (100 mL) and concentrated to half volume.
The resulting mixture was cooled to 0° C. and ammonium hydroxide (60 mL, 437 mmol) was added slowly via syringe.
The reaction was stirred for 10 min at room temperature, diluted with EtOAc (200 mL) and washed with water (3*100 mL).
The organic layer was dried over anhydrous sodium sulfate and concentrated.
The residue was suspended in 9:1 heptane/EtOAc (300 mL) and filtered.
The filtered solids were collected and the remaining mother liquor was partially evaporated to half volume, cooled to 0° C., and filtered.
The two crops of filtered solids were combined to provide 2,5,6-trichloronicotinamide (Intermediate P, 46.2 g, 92% yield).
References:
Amgen Inc.;ALLEN, John Gordon;LANMAN, Brian Alan;CHEN, Jian;REED, Anthony B.;CEE, Victor J.;LIU, Longbin;LOPEZ, Patricia;WURZ, Ryan Paul;NGUYEN, Thomas T.;Booker, Shon;ALLEN, Jennifer Rebecca;CHU-MOYER, Margaret;AMEGADZIE, Albert;CHEN, Ning;GOODMAN, Clifford;LOW, Jonathan D.;MA, Vu Van;MINATTI, Ana Elena;NISHIMURA, Nobuko;PICKRELL, Alexander J.;WANG, Hui-Ling;SHIN, Youngsook;SIEGMUND, Aaron C.;YANG, Kevin C.;TAMAYO, Nuria A.;WALTON, Mary;XUE, Qiufen US2019/374542, 2019, A1 Location in patent:Paragraph 0784-0785

58584-88-6
12 suppliers
inquiry

142266-62-4
82 suppliers
$121.00/100mg
40381-92-8
101 suppliers
inquiry

142266-62-4
82 suppliers
$121.00/100mg

52465-59-5
18 suppliers
inquiry

142266-62-4
82 suppliers
$121.00/100mg
29553-51-3
43 suppliers
$47.00/100mg

142266-62-4
82 suppliers
$121.00/100mg