
2-(5-BroMopyridin-3-yl)oxazole synthesis
- Product Name:2-(5-BroMopyridin-3-yl)oxazole
- CAS Number:342600-96-8
- Molecular formula:C8H5BrN2O
- Molecular Weight:225.04
![Ethanamine, N-[(5-bromo-3-pyridinyl)methylene]-2,2-dimethoxy-](/CAS/20210305/GIF/342600-95-7.gif)
342600-95-7
0 suppliers
inquiry

342600-96-8
13 suppliers
inquiry
Yield:342600-96-8 100%
Reaction Conditions:
Stage #1: C10H13BrN2O2with sulfuric acid;phosphorus pentoxide at 20 - 100;
Stage #2: with ammonia in water at 0; pH=8;
Steps:
43.B
To the acetal obtained in Step A (361.0 mg; 1.32 mmol) was added with cooling to 0° C. concentrated H2SO4 (7 mL). Next, P2O5 (487.0 mg; 1.72 mmol) was added as a solid and the whole was heated to 100° C. for 30 minutes. The reaction mixture was allowed to reach ambient temperature and stirred overnight. The next day, the contents of the reaction were poured onto ice and concentrated NH4OH was added with cooling to 0° C. until the reaction mixture was approximately pH=8. The aqueous layer was extracted several times with CHCl3. The organic layer was then washed with brine. Drying (MgSO4), filtration and removal of the solvent in vacuo provided quantitative yield of the desired oxazole.
References:
US6642237,2003,B1 Location in patent:Page/Page column 152-153
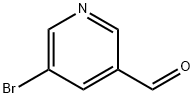
113118-81-3
285 suppliers
$5.00/250mg

342600-96-8
13 suppliers
inquiry