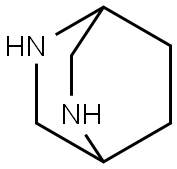
2,5-Diazabicyclo[2.2.2]octane synthesis
- Product Name:2,5-Diazabicyclo[2.2.2]octane
- CAS Number:658-24-2
- Molecular formula:C6H12N2
- Molecular Weight:112.17
![2,5-Diazabicyclo[2.2.2]octane-3,6-dione](/CAS/20150408/GIF/1004-98-4.gif)
1004-98-4
29 suppliers
$321.00/250mg
![2,5-Diazabicyclo[2.2.2]octane](/CAS/GIF/658-24-2.gif)
658-24-2
26 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:2,5-diazabicyclo<2.2.2>octane-3,6-dione with lithium aluminium tetrahydride in tetrahydrofuran for 16 h;Heating / reflux;
Stage #2: with sodium hydroxide;water in tetrahydrofuran at 20; for 1 h;
Steps:
129
Example 129. 2,5-Diazabicyclo [2.2.2] octane (129); [0515] A soxhlet extractor thimble was charged with diketopiperazine 128 (100 mg,0.71 mmol), then placed inside a soxhlet suspended above a flask containing a solution of LAH (4.3 mL, 4.30 mmol, 1.0 M in THF) in THF (100 mL). The system was then heated at reflux for 16 h. Once cooled to 0 0C, the reaction mixture was quenched via the addition of water (200 μL), 4 M NaOH (200 μL) then more water (600 μL). On stirring the resultant suspension at rt for 1 h, the mixture was dried (Na2SO4), filtered, and the filter cake washed with THF (100 mL). The combined filtrates were evaporated to afford
References:
PANACOS PHARMACEUTICALS, INC.;NITZ, Theodore, J.;SALZWEDEL, Karl;FINNEGAN, Catherine;WILD, Carl;BRUNTON, Shirley;FLANAGAN, Stuart;MONTALBETTI, Christian;COULTER, Thomas, Stephen;KIMBER, Marc;MAGARACI, Filippo;JOHNSTON, David WO2008/134035, 2008, A1 Location in patent:Page/Page column 195-196
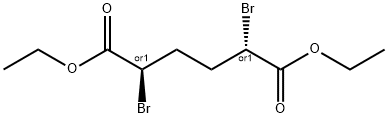
54221-37-3
36 suppliers
$77.00/5g
![2,5-Diazabicyclo[2.2.2]octane](/CAS/GIF/658-24-2.gif)
658-24-2
26 suppliers
inquiry