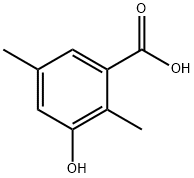
2,5-DIMETHYL-3-HYDROXY BENZOIC ACID synthesis
- Product Name:2,5-DIMETHYL-3-HYDROXY BENZOIC ACID
- CAS Number:27023-06-9
- Molecular formula:C9H10O3
- Molecular Weight:166.17
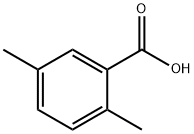
610-72-0
230 suppliers
$5.00/1g

27023-06-9
25 suppliers
inquiry
Yield:27023-06-9 44%
Reaction Conditions:
Stage #1: 2,5-dimethylbenzoic acidwith oleum;sulfuric acid at 110; for 2 h;
Stage #2: with sodium hydroxide in water at 0;
Stage #3: with hydrogenchloride;potassium hydroxidemore than 3 stages;
Steps:
2, 5-dimethyl-benzoic acid (1) (20 G, 133 MMOL) was dissolved in concentrated H2SO4 (30 mL) and fuming H2SO4 (20% SO3, 70 mL). The reaction mixture was heated to 110 °C for 2 hours. After cooling, the solution was poured carefully into a beaker of ice H2O (400 mL) and was then neutralized with 20% aqueous NAOH (400 mL). The H20 was partially removed in vacuo until a white salt mixture started to form. The solid was collected on a sintered-glass funnel and was then dried in a . vacuum oven. The dried salt mixture was placed in a ceramic crucible with KOH (160 g) and was melted together using A butane torch for 0.5 h. After cooling, the fused solid was dissolved in H2O (300 mL) and acidified with concentrated HCI (300 mL). The product was extracted from the aqueous solution with EtOAc (3 x 200 mL). The combined organic layers were washed with brine (100 mL) and dried over MGS04. The solvents were removed in vacuo and the solid residue was recrystallized with 20% ETOAC/CHC13 four times to afford 3-hydroxy-2, 5-dimethyl-benzoic acid (2) as a light brown solid (9.8 g, 44%) 'H NMR (ACETONE-D6) 10.93 (br s, 1H), 8.34 (br s, 1H), 7.20 (s, 1H), 6. 86 (s, 1H), 2.37 (s, 3H), 2.24 (s, 3H). References-Fujiwara, A. N.; Acton, E. M. , Can. J. CHEM., 1970,48, 1346-1349. Charlesworth, E. H.; Levene, L., Can. J. Chem., 1963,41, 1071-1077.
References:
WO2005/26114,2005,A1 Location in patent:Page/Page column 88-89