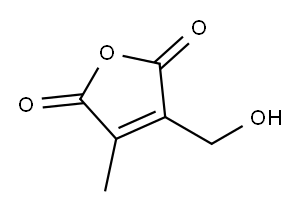
2,5-Furandione, 3-(hydroxymethyl)-4-methyl- (9CI) synthesis
- Product Name:2,5-Furandione, 3-(hydroxymethyl)-4-methyl- (9CI)
- CAS Number:245124-18-9
- Molecular formula:C6H6O4
- Molecular Weight:142.11
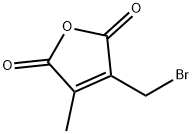
98453-81-7

245124-18-9
General procedure for the synthesis of 3-(bromomethyl)-4-methylfuran-2,5-dione from 3-(bromomethyl)-4-methylfuran-2,5-dione: To 2-bromomethyl-3-methylmaleic anhydride (1.25 g, 6.10 mmol) was added 5 M aqueous sodium hydroxide (4 mL) at room temperature and the mixture was stirred for 12 hours. The reaction mixture was adjusted to pH 1 with 5 M hydrochloric acid (5 mL), sodium chloride was added and extracted three times with ethyl acetate. The combined organic layers were concentrated under reduced pressure and the resulting residue was purified by silica gel column chromatography (eluent: hexane-ethyl acetate, 2:1) to afford 227 mg of 3-(hydroxymethyl)-4-methylfuran-2,5-dione as an oil (yield: 26%).1H NMR (CDCl3) δ 2.21 (t, J = 1.1Hz, 3H), 4.63 (q, J = 1.1Hz, 2H).

98453-81-7
21 suppliers
inquiry

245124-18-9
15 suppliers
inquiry
Yield:245124-18-9 355 mg
Reaction Conditions:
Stage #1: 2-(bromomethyl)-3-methyl maleic anhydratewith potassium hydroxide in water at 20; for 5 h;Cooling with ice;
Stage #2: with sulfuric acid;sodium chloride at 20; for 0.5 h;
Steps:
7.NN.OO OO. Intermediate 21
Intermediate Compound 2 (1 g, 4.9 mmol) was added to an ice cold solution of 4N aq. KOH (5 ml), and the mixture was stirred at room temperature for 5 hrs. The mixture was slowly acidified with 6N H2SO4 (5 ml), then saturated with solid NaCl and stirred at room temperature for 30 min. The aqueous layer was extracted with ethyl acetate and the organic layer was washed with brine and dried. The organic layer was concentrated in vacuo and the concentrate was applied to silica gel (PE:EA=1:1) to furnish 355 mg of product (intermediate 21).
References:
WO2021/194954,2021,A1 Location in patent:Paragraph 00363; 00399
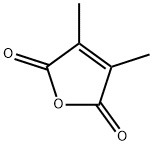
766-39-2
250 suppliers
$10.00/5g

245124-18-9
15 suppliers
inquiry